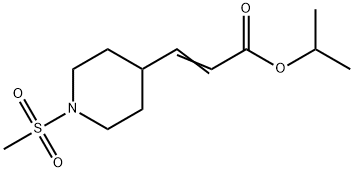
3-(1-Methanesulfonyl-piperidin-4-yl)-acrylic acid isopropyl ester synthesis
- Product Name:3-(1-Methanesulfonyl-piperidin-4-yl)-acrylic acid isopropyl ester
- CAS Number:937282-79-6
- Molecular formula:C12H21NO4S
- Molecular Weight:275.36
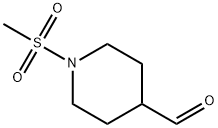
241134-35-0
28 suppliers
$90.00/50mg
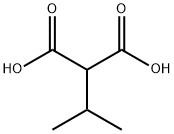
601-79-6
95 suppliers
$21.00/1g

937282-79-6
6 suppliers
inquiry
Yield:937282-79-6 59%
Reaction Conditions:
Stage #1: 1-(methylsulfonyl)-4-piperidinecarboxaldehyde;isopropylmalonic acid;piperidine in toluene at 85 - 95;
Stage #2: with hydrogenchloride in water;toluene at 40 - 50; for 0.25 h;
Steps:
5
Preparation 5 Preparation of /so-propyl 3-(l-methanesulfonylpiperidin-4-yl)propenoate(l-Methanesulfonylpiperidin-4-yl)methanal (1 mol eq) was charged to a reaction vessel followed by a line wash of toluene (11 rel vol). Piperidine (0.1 mol eq) was charged to the vessel followed by a line wash of toluene (0.5 rel vol), and the reaction mixture heated to between 85 and 950C. A solution of the wo-propyl malonic acid (1.25 mol eq) in toluene (prepared as described above) was added in 10 approximately equal portions over 6 to 8 hours and the reaction mixture was stirred at to between 85 and 950C until it reached completion. The reaction mixture was then cooled to between 40 and 5O0C and HCl (0.5M, 3 rel vol) was added to the reaction maintaining the temperature between 40 and 500C. After stirring for at least 15 minutes the phases were separated. Sodium bicarbonate (0.5M, 3 rel vol) was added to the organic phase, still maintaining the temperature between 40 and 5O0C. The 2-phase mixture was stirred for at least 15 minutes before separating the phases and washing the organic phase with water (3 rel vol). The organic phase was then concentrated to approximately 16 rel vols by vacuum distillation at between 40 and 500C. Toluene (3.5 rel vol) was charged, the solution clarified at between 40 and 5O0C and then concentrated to approximately 7 rel vol by vacuum distillation. The mixture was then cooled to between 0 and 100C and stirred for at least 60 minutes at this temperature before isolating the sub-titled compound by filtration and washing the residue with toluene (2 rel vol) at between 0 and 100C. The solid was dried to leave the sub-titled compound in approximately 59% yield.1H NMR (400 MHz5 CDCl3) δ 6.87 (dd, J= 15.8, 6.5 Hz, IH), 5.81 (dd, J= 15.8, 0.9 Hz, IH), 5.07 (quintet, J= 6.2 Hz, IH), 3.82 (d, J= 12.0 Hz, 2H), 2.79 (s, 3H), 2.74 (td, J=
References:
WO2007/57643,2007,A1 Location in patent:Page/Page column 9-10

241134-35-0
28 suppliers
$90.00/50mg

56766-77-9
13 suppliers
inquiry

937282-79-6
6 suppliers
inquiry
2033-24-1
643 suppliers
$6.00/25g

937282-79-6
6 suppliers
inquiry

217487-18-8
20 suppliers
$45.00/50mg

937282-79-6
6 suppliers
inquiry
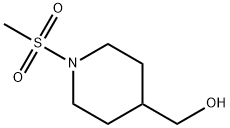
241134-34-9
21 suppliers
$188.00/250mg

937282-79-6
6 suppliers
inquiry