
2-(CHROMAN-8-YL)-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE synthesis
- Product Name:2-(CHROMAN-8-YL)-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE
- CAS Number:937591-99-6
- Molecular formula:C15H21BO3
- Molecular Weight:260.14
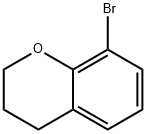
3722-78-9
52 suppliers
$90.00/100mg
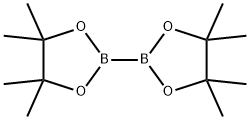
73183-34-3
563 suppliers
$6.00/5g

937591-99-6
11 suppliers
inquiry
Yield: 56%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,2-dimethoxyethane at 150; for 0.25 h;Microwave irradiation;
Steps:
10
Example 10 8-(4,4,5,5-Tetramethyl-l,3,2-dioxaborolan-2-yl)chromane 8-Bromochromane (described in Gerard H. Thomas et al. Tetrahedron. Lett. 1998, 39, 2219-2222, 426 mg, 2 mmol), 4,4,4',4I,5,5,5',51-octamethyl-2,2'-bi-l,3,2-dioxaborolane (609 mg, 2.4 mmol), [l,r-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane adduct (50 mg, 0.06 mmol), potassium acetate (590 mg, 6 mmol) and 1,2- dimethoxyethan (3 mL) was irradiated in a microwave at 150 0C for 15 min. When cooled to ambient temperature the mixture was diluted with water (5 mL) and extracted with diethyl ether (3 x 20 mL). The crude product was purified by flash chromatography, using dichloromethane/methanol (95:5) as the eluent, to give 290 mg (56% yield) of the title compound. 1H-NMR (400 MHz5 OMSO-d6): δ 7.32 (dd, J = 7.3, 1.5 Hz, IH), 7.12 (dd, J = 7.4, 1.6 Hz, IH), 6.76 (t, J = 7.4 Hz5 IH)54.12 (t, J = 5.0 Hz52H)5 2.71 (t, J = 6.5 Hz52H), 1.92-1.84 (m, 2H), 1.25 (s, 12H).
References:
ASTRAZENECA AB;ASTEX THERAPEUTICS LTD WO2007/73284, 2007, A1 Location in patent:Page/Page column 46