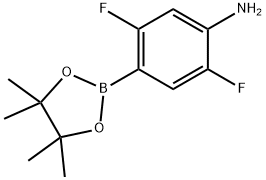
2,5-difluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenamine synthesis
- Product Name:2,5-difluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenamine
- CAS Number:939807-75-7
- Molecular formula:C12H16BF2NO2
- Molecular Weight:255.07
112279-60-4
166 suppliers
$12.00/5g

73183-34-3
569 suppliers
$6.00/5g

939807-75-7
15 suppliers
inquiry
Yield:939807-75-7 85%
Reaction Conditions:
with tris(dibenzylideneacetone)dipalladium(0) chloroform complex;potassium acetate;tricyclohexylphosphine in 1,4-dioxane at 120;
Steps:
36.1 Step 1: 2,5-Difluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
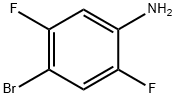
A flask was charged with Pd2(dba)3.CHCl3 (45 mg, 0.04 mmol) and tricyclohex- ylphosphine (55 mg, 0.20 mmol). Degased 1,4-Dioxane (10 mL) was added and the resulting mixture was stirred 25 minutes at rt before addition of bis(pinacolato)diboron (700 mg, 2.76 mmol), potassium acetate (715 mg, 7.21 mmol) and 4-Bromo-2,5-difluoroaniline (500 mg, 2.40 mmol) in that order. The reaction mixture was stirred overnight at 120 oC then diluted with EtOAc, filtered through a pad of celite and concentrated under reduced pressure. The crude material was purified by flash chromatography through silica gel (0 - 100% (0994) CH2Cl2/heptanes) to provide the title compound (524 mg, 85% yield). LCMS (ESI) [M+H]+ = 256.3.
References:
WO2020/172093,2020,A1 Location in patent:Paragraph 0700-0701