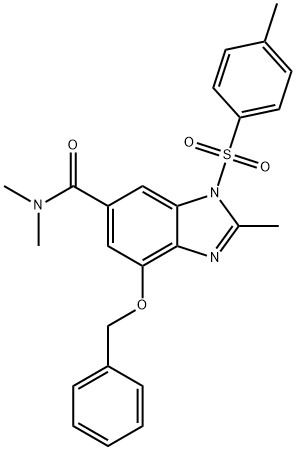
4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide synthesis
- Product Name:4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide
- CAS Number:942195-85-9
- Molecular formula:C25H25N3O4S
- Molecular Weight:463.55
![4-(benzyloxy)-6-bromo-2-methyl-1-tosyl-1H-benzo[d]imidazole](/CAS/20180531/GIF/942195-88-2.gif)
942195-88-2
4 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

124-40-3
500 suppliers
$18.00/100ml
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry
Yield:942195-85-9 42%
Reaction Conditions:
tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran at 65; under 760.051 Torr; for 32 h;
Steps:
8.3
A mixture of 4-(benzyloxy)-6-bromo-2-methyl-1-[(4-methylphenyl)sulfonyl]-1H-benzimidazole (53.0 g, 112 mmol, Step 2) and tetrakis(triphenylphosphine)palladium (25.9 g, 22.4 mmol) in 2 mol/L dimethylamine tetrahydrofuran solution (580 mL) was stirred at 65°C under carbon monoxide gas (1 atm) for 32 hours. The mixture was cooled to room temperature, and diluted with ethyl acetate. The organic 5 mixture was washed with saturated ammonium chloride aqueous solution and brine, dried over magnesium sulfate and concentrated in vacuum. The residue was purified by column chromatography on silica gel eluting with hexane/ethyl acetate (gradient elution from 1 :2 to 1 :3) to afford the title compound as a white solid (21.8 g, 42%). 1H NMR (CDCI3, 270 MHz) δ: 7.80 (d, J = 8.1 Hz, 2H), 7.70 (s, 1H), 7.45 (d, J = 8.1 Hz, 2H), 7.40-7.22 (m,10 5H), 6.86 (s, 1 H), 5.32 (s, 2H), 3.11 (br. s, 3H), 2.89 (br. s, 3H), 2.81 (s, 3H), 2.40 (s, 3H) ppm. MS (ESI) m/z: 464 (M+H)+.
References:
WO2008/35195,2008,A1 Location in patent:Page/Page column 44-45
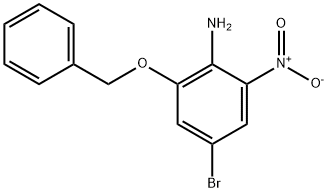
713530-47-3
20 suppliers
inquiry
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry
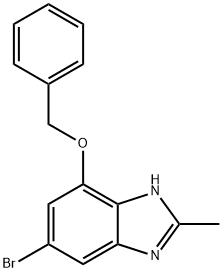
774582-60-4
17 suppliers
inquiry
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry
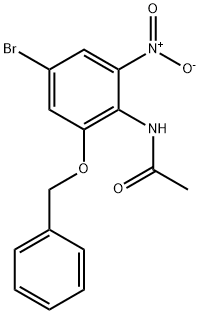
942195-80-4
7 suppliers
inquiry
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry
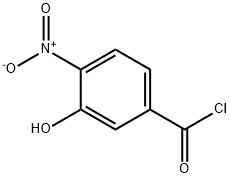
36852-58-1
5 suppliers
inquiry
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry