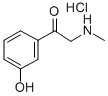
1-(3-Hydroxyphenyl)-2-(methylamino)ethanone hydrochloride synthesis
- Product Name:1-(3-Hydroxyphenyl)-2-(methylamino)ethanone hydrochloride
- CAS Number:94240-17-2
- Molecular formula:C9H12ClNO2
- Molecular Weight:201.65
2491-37-4
152 suppliers
$29.00/1g

74-89-5
7 suppliers
$13.44/25ML

94240-17-2
95 suppliers
$110.00/10mg
Yield:94240-17-2 48%
Reaction Conditions:
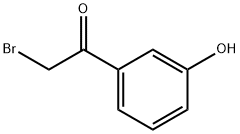
Stage #1:2-bromo-1-(3-hydroxyphenyl)ethanone;methylamine in tetrahydrofuran at 5; for 2.5 h;ice-salt bath;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0; for 0.5 h;
Steps:
2
The title compound was synthesized following a modified literature procedure (JP-A 63-192744). The whole procedure was performed under nitrogen atmosphere. A solution of methylamine (85 mL, 0.70 mol) in 250 mL tetrahydrofuran (THF) was cooled in an ice-salt bath. A solution of 2-bromo-3'-hydroxyacetophenone (42.5 g, 0.23 mol) in 750 mL of THF was added dropwise into the above solution within a period of 30 minutes. The temperature was kept below 5 °C, and after complete addition stirring was continued for 2 hours. At the beginning of addition, the solution became light yellow. The reaction was monitored by HPLC and TLC (eluent: CH2Cl2/Me0H, v:v = 10:1). The mixture was then concentrated under
References:
LONZA AG WO2008/77560, 2008, A1 Location in patent:Page/Page column 9-10
71786-67-9
168 suppliers
$99.00/5g

94240-17-2
95 suppliers
$110.00/10mg