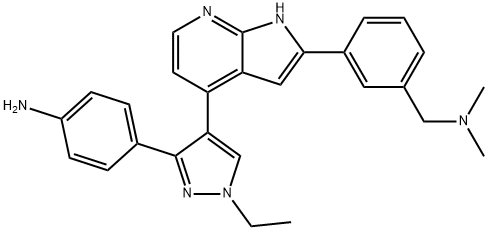
3-[4-[3-(4-Aminophenyl)-1-ethyl-1H-pyrazol-4-yl]-1H-pyrrolo[2,3-b]pyridin-2-yl]-N,N-dimethylbenzenemethanamine synthesis
- Product Name:3-[4-[3-(4-Aminophenyl)-1-ethyl-1H-pyrazol-4-yl]-1H-pyrrolo[2,3-b]pyridin-2-yl]-N,N-dimethylbenzenemethanamine
- CAS Number:942919-53-1
- Molecular formula:C27H28N6
- Molecular Weight:436.55
![3-[4-[1-Ethyl-3-(4-nitrophenyl)-1H-pyrazol-4-yl]-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-2-yl]-N,N-dimethylbenzenemethanamine](/CAS/GIF/942920-68-5.gif)
942920-68-5
8 suppliers
inquiry
![3-[4-[3-(4-Aminophenyl)-1-ethyl-1H-pyrazol-4-yl]-1H-pyrrolo[2,3-b]pyridin-2-yl]-N,N-dimethylbenzenemethanamine](/CAS/GIF/942919-53-1.gif)
942919-53-1
9 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-[3-(4-nitrophenyl)-1-ethyl-1H-pyrazol-4-yl]-2-[3-(dimethylaminomethyl)phenyl]-1-phenylsulfonyl-1H-pyrrolo[2,3-b]pyridinewith acetic acid;zinc at 20; for 1.08333 h;
Stage #2: with sodium hydroxide in water at 70; for 8 h;
Steps:
102
Intermediate 102; 4-[3-(4-aminophenyl)-1-ethyl-1H-pyrazol-4-yl]-2-[3-(dimethylaminomethyl)phenyl]-1H-pyrrolo[2,3-b]pyridine; To a stirred solution of 4-[3-(4-nitrophenyl)-1-ethyl-1H-pyrazol-4-yl]-2-[3-(dimethylaminomethyl)phenyl]-1-phenylsulfonyl-1H-pyrrolo[2,3-b]pyridine (1.1 mmol) in HOAc (20 mL) was added portionwise zinc dust (8.0 mmol) over 5 minutes. The reaction was stirred at room temperature for 1 h, filtered through a pad of Celite, rinsed with acetic acid, and concentrated to dryness under vacuum. The residue was re-evaporated several times from methanol/toluene to remove the excess acetic acid, taken up in methanol (35 mL) and treated with 6 N sodium hydroxide (1.5 mL). The reaction mixture was stirred and heated at 70 oC for 8 h. After cooling to room temperature the reaction was concentrated under vacuum, triturated with cold water, filtered, washed with water, and dried under vacuum to give the crude product as a pale orange solid: ESMS [M+H]+: 437.2
References:
US2007/149561,2007,A1 Location in patent:Page/Page column 93
![4-(4-(2-(3-((diMethylaMino)Methyl)phenyl)-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-4-yl)-1-ethyl-1H-pyrazol-3-yl)aniline](/CAS/20130318/GIF/CB52623970.gif)
1415560-01-8
2 suppliers
inquiry
![3-[4-[3-(4-Aminophenyl)-1-ethyl-1H-pyrazol-4-yl]-1H-pyrrolo[2,3-b]pyridin-2-yl]-N,N-dimethylbenzenemethanamine](/CAS/GIF/942919-53-1.gif)
942919-53-1
9 suppliers
inquiry