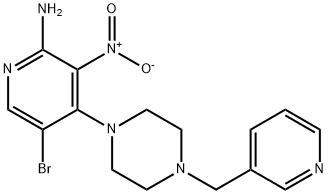
5-Bromo-3-nitro-4-(4-(pyridin-3-ylmethyl)piperazin-1-yl)pyridin-2-amine synthesis
- Product Name:5-Bromo-3-nitro-4-(4-(pyridin-3-ylmethyl)piperazin-1-yl)pyridin-2-amine
- CAS Number:942948-39-2
- Molecular formula:C15H17BrN6O2
- Molecular Weight:393.24
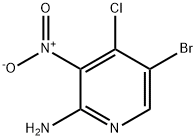
942947-95-7
97 suppliers
$25.00/250mg
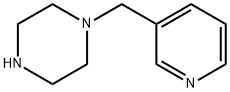
39244-80-9
110 suppliers
$33.00/1g

942948-39-2
4 suppliers
inquiry
Yield:942948-39-2 82%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in isopropyl alcohol at 45; for 18 h;
Steps:
27 5-Bromo-3-nitro-4-(4-(pyridin-3-ylmethyl)piperazin-1-yl)pyridin-2-amine
To a mixture of 5-bromo-4-chloro-3-nitro-pyridin-2-ylamine (0.126 g, 0.50 mmol) and isopropanol (9 ml) was added 1-[(3-pyridyl)-methyl]-piperazine (0.097 g, 0.55 mmol) followed by diisopropylethylamine (0.10 ml, 0.57 mmol). The reaction mixture was heated at 45° C. for 18 h, then allowed to cool to room temperature. The precipitate was collected by filtration and washed with isopropanol and diethyl ether. The title compound was thus obtained as a yellow solid (0.160 g, 82%); 1H-NMR (500 MHz, DMSO-d6) 3.05 (br s, 4H, piperazine N(CH2)2), 3.56 (s, 2H, NCH2), 7.02 (s, 2H, NH2), 7.36 (dd, J=7.80, 4.75 Hz, 1H, pyridyl 5-H), 7.74 (dt, J=7.80, 1.70 Hz, 1H, pyridyl 4-H), 8.16 (s, 1H, 6-H), 8.47 (dd, J=4.75, 1.60 Hz, 1H, pyridyl 6-H), 8.50 (d, J=1.65 Hz, 1H, pyridyl 2-H);LC (Method B)-MS (ESI, m/z): Rt=1.79 min-393, 395 [(M+H)+, Br isotopic pattern].
References:
US2009/247507,2009,A1 Location in patent:Page/Page column 22