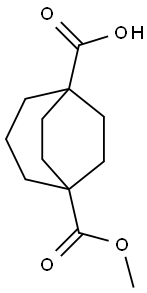
5-(methoxycarbonyl)bicyclo[3.2.2]nonane-1-carboxylic acid synthesis
- Product Name:5-(methoxycarbonyl)bicyclo[3.2.2]nonane-1-carboxylic acid
- CAS Number:942999-81-7
- Molecular formula:C12H18O4
- Molecular Weight:226.27
![1,5-dimethyl bicyclo[3.2.2]nonane-1,5-dicarboxylate](/CAS/20180702/GIF/942999-92-0.gif)
942999-92-0
23 suppliers
$334.00/250mg
![5-(methoxycarbonyl)bicyclo[3.2.2]nonane-1-carboxylic acid](/CAS/20180702/GIF/942999-81-7.gif)
942999-81-7
16 suppliers
$45.00/10mg
Yield:942999-81-7 49%
Reaction Conditions:
Stage #1: dimethyl bicyclo[3.2.2]nonane-1,5-dicarboxylatewith methanol;barium dihydroxide;water at 20;
Stage #2: with hydrogenchloride;water pH=2;
Steps:
19
Intermediate 19: Bicvclof3.2.21nonane-l,5-diearboxylie acid monomethyl esterTo a stirred solution of dimethyl bicyclo[3.2.2]nonane-l,5-dicarboxylate (2.02 g, 8.41 mmol), in methanol (20 mL) and water (4 mL) was added barium hydroxide octahydrate (1.33 g, 4.20 mmol). The reaction mixture was stirred vigorously at ambient temperature overnight.. The reaction mixture was stirred vigorously at ambient temperature overnight. The reaction mixture was diluted with water (20 mL) and washed with isohexane (2 x 100 mL), then the aqueous phase was acidified with 2M HCl to pH 2 and extracted into EtOAc (2 x 100 mL). The organic extracts were combined, dried (MgSO4) and concentrated to leave the title compound (931mg, 4.11 mmol, 49%) as a clear colourless gum which slowly formed a white solid; 1H NMR (CDCl3) δ 1.72 - 1.75 (m, 2H), 1.87 - 1.92 (m, 12H), 3.65 (s, 3H); GC-MS EI m/e M+ 226.
References:
WO2007/71966,2007,A1 Location in patent:Page/Page column 51