
2-chloro-1-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)ethanone synthesis
- Product Name:2-chloro-1-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)ethanone
- CAS Number:943126-72-5
- Molecular formula:C11H10ClFO2
- Molecular Weight:228.65
![Sulfur, [2-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)-2-oxoethylidene]dimethyloxo-](/CAS/20200119/GIF/1017878-51-1.gif)
1017878-51-1
0 suppliers
inquiry

943126-72-5
13 suppliers
inquiry
Yield: 78%
Reaction Conditions:
Stage #1:dimethylsulfoxonium-2-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)-2-oxoethylide with methanesulfonic acid;lithium chloride in tetrahydrofuran at 0 - 70; for 18.8333 h;
Stage #2: with water;sodium hydrogencarbonate in tetrahydrofuran
Steps:
6
Example 6
References:
ZACH SYSTEM S.P.A. WO2008/40528, 2008, A2 Location in patent:Page/Page column 12-13
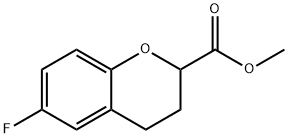
874649-82-8
84 suppliers
$9.00/250mg

74-97-5
2 suppliers
$15.00/25 g

943126-72-5
13 suppliers
inquiry
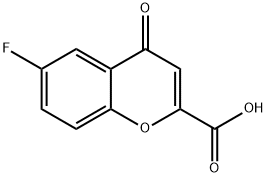
99199-59-4
140 suppliers
$34.46/1g

943126-72-5
13 suppliers
inquiry
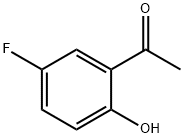
394-32-1
345 suppliers
$9.00/5g

943126-72-5
13 suppliers
inquiry
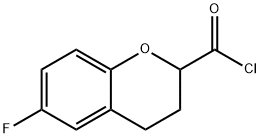
850896-51-4
3 suppliers
inquiry

943126-72-5
13 suppliers
inquiry