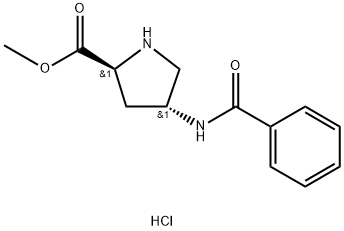
L-Proline, 4-(benzoylaMino)-, Methyl ester, hydrochloride (1:1), (4R)- synthesis
- Product Name:L-Proline, 4-(benzoylaMino)-, Methyl ester, hydrochloride (1:1), (4R)-
- CAS Number:943134-38-1
- Molecular formula:C13H17ClN2O3
- Molecular Weight:284.74
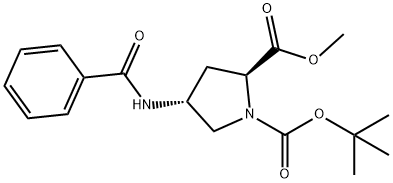
943134-37-0
9 suppliers
inquiry

943134-38-1
5 suppliers
inquiry
Yield:943134-38-1 99%
Reaction Conditions:
with hydrogenchloride in diethyl ether at 20; for 45.0833 h;
Steps:
3
(2S,4R)-1-tert-Butyl-2-methyl-4-benzamidopyrrolidine-1,2-dicarboxylate (60.19 g, contains 5.6% EtOAc; 0.1631 mol) was dissolved in Et2O (100 mL), and the solvent was evaporated under vacuum to remove residual EtOAc. The residual oil was dissolved in Et2O (100 mL). 2N HCl solution in Et2O (700 mL) was added (mild exotherm; precipitation commenced after about 5 min). The mixture was stirred at ambient temperature for 21 h. At that point, 200 mL of 2N HCl solution in Et2O was added, and the mixture was stirred for additional 24 h. The precipitate was filtered, washed with 500 mL of diethyl ether, and dried in vacuum at ambient temperature for 24 h to afford 46.03 g of (2S,4R)-methyl 4-benzamidopyrrolidine-2-carboxylate hydrochloride (99% yield). 1H NMR (CD3OD, δ, ppm): 7.91-7.84 (m, 2 H), 7.6-7.44 (m, 3H), 4.78 (t, J=8.5 Hz, 1 H), 4.69-4.59 (m, 1 H), 3.77 (dd, J=12, 6.6 Hz, 1 H), 3.52 (dd, J=12, 5 Hz, 1 H), 2.67-2.5 (m, 2 H). MS (m/z, positive ESI, for M+H): 249.
References:
US2007/149460,2007,A1 Location in patent:Page/Page column 34
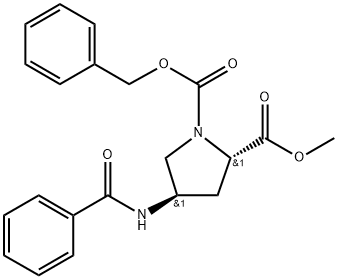
1035596-37-2
1 suppliers
inquiry

943134-38-1
5 suppliers
inquiry