
(2S,4R)-1-(2-Aminoacetyl)-4-benzamidopyrrolidine-2-carboxylic acid synthesis
- Product Name:(2S,4R)-1-(2-Aminoacetyl)-4-benzamidopyrrolidine-2-carboxylic acid
- CAS Number:943134-39-2
- Molecular formula:C14H17N3O4
- Molecular Weight:291.3
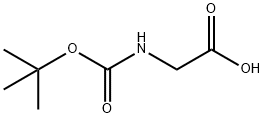
4530-20-5
554 suppliers
$5.00/5 g
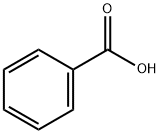
65-85-0
1376 suppliers
$10.00/25 g
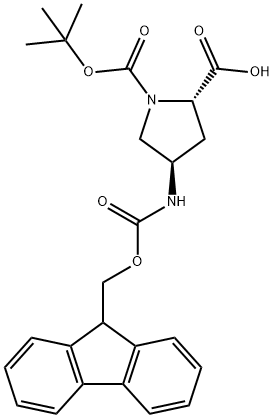
176486-63-8
104 suppliers
$33.00/100mg

943134-39-2
44 suppliers
inquiry
Yield:-
Steps:
2
(2S,4R) 1-(2-Amino-acetyl)-4-benzoylamino-pyrrolidine-2-carboxylic acid PAM-resin (Advanced Chemtech) was swelled in DMF, washed with 5% Triethyl amine (TEA) in DMF and washed with DMF until no yellow color could be detected after adding Dhbt-OH to the drained DMF. (2S,4R) Boc-4Amp(Fmoc)-OH was coupled as symmetrical anhydride as follows. 3eq (2S,4R) Boc-4Amp(Fmoc)-OH was dissolved in DCM and cooled to 0° C. DIC (1.5 eq.) was added and the reaction continued for 10 minutes. The solvent was removed in vacuo and the residue dissolved in DMF. The solution was immediately added to the resin followed by 0.1 eq. of DMAP. The coupling was continued over night. Excess coupling reagent was removed by washing with DMF. Deprotection of the Fmoc group was performed by treatment with 20% piperidine in DMF (1×5 and 1×10 min.), followed by washing with DMF until no yellow colour could be detected after addition of Dhbt-OH to the drained DMF. Coupling of benzoic acid was carried out as follows. 3 eq. benzoic acid was dissolved in DMF together with 3 eq. HOBt and 3 eq. DIC and then added to the resin. The coupling was continued over night. Excess coupling reagent was removed by washing with DMF. Prior to the deprotection of the Boc group the resin was treated with DCM. Deprotection of the Boc group was performed by treatment with 50% TFA in DCM v/v (2×2 min, 1×30 min) followed by washing with DCM and then with DMF and then treatment with 5% DIEA in DMF v/v and finally followed by washing with DMF. Coupling of Boc-Gly-OH was carried out as follows. 3 eq. Boc-Gly-OH was dissolved in DMF together with 3 eq. HOBt and 3 eq. DIC and then added to the resin. The coupling was continued 2 hours. Excess coupling reagent was removed by washing with DMF. The coupling was repeated and continued over night. Before cleavage of the peptide from the solid support the peptide resin was washed with DCM and then with ether and finally dried under vacuum. Cleavage of the dipeptide from the PAM-Resin was carried out as follows. The peptide-resin was treated with trifluoroacetic acid (TFA, Riedel-de Häen) and after 10 min a volume corresponding to 10% of the TFA total volume of trifluoromethanesulfonic acid (TFMSA, Aldrich) was added at room temperature and the reaction was continued for 2 hours. The filtered resins were washed with TFA. The raw material was precipitated from the TFA-solution by adding diethylether. The raw material was collected as a brown oil. The ether solution was further extracted with water and the water phase was evaporated. The total amount of raw material was purified using prep. HPLC (Vydac C18-column): Buffer A: 0.1% TFA in Water; Buffer B: 90% AcCN; 0.1% TFA; 9.9% Water. Flow: 35ml/min. Gradient: 0-47 min 100% A to 75% A (Linear). HPLC purity: 99%. MS: calculated M+H=291.12. found M+H=291.7.
References:
Larsen, Bjarne Due;Petersen, Jorgen Soberg;Haugan, Ketil Jorgen;Butera, John A.;Hennan, James K.;Kerns, Edward H.;Piatnitski, Evgueni Lvovich US2007/149460, 2007, A1 Location in patent:Page/Page column 33-34
![L-Proline, N-[(1,1-diMethylethoxy)carbonyl]glycyl-4-(benzoylaMino)-, (4R)-](/CAS/20150408/GIF/943134-34-7.gif)
943134-34-7
7 suppliers
inquiry

943134-39-2
44 suppliers
inquiry