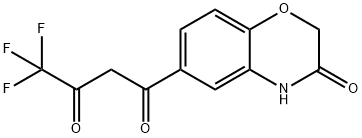
4,4,4-TRIFLUORO-1-(3-OXO-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZIN-6-YL)BUTANE-1,3-DIONE synthesis
- Product Name:4,4,4-TRIFLUORO-1-(3-OXO-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZIN-6-YL)BUTANE-1,3-DIONE
- CAS Number:943994-23-8
- Molecular formula:C12H8F3NO4
- Molecular Weight:287.1914

26518-71-8
81 suppliers
$45.00/10mg
![4,4,4-TRIFLUORO-1-(3-OXO-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZIN-6-YL)BUTANE-1,3-DIONE](/CAS/20180601/GIF/943994-23-8.gif)
943994-23-8
16 suppliers
inquiry
Yield:943994-23-8 80%
Reaction Conditions:
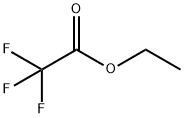
Stage #1: ethyl trifluoroacetate,with sodium hydride in tetrahydrofuran;
Stage #2: 6-acetyl-4H-benzo[1,4]oxazin-3-onewith dibenzo-18-crown-6 in tetrahydrofuran;ethanol; for 16 h;Heating / reflux;
Steps:
71.1
To a mixture of NaH (2.51 g, 105 mmol) in THF (100 mL) was carefully added ethyl 2, 2, 2-trifluoroacetate (12.5 mL,
References:
WO2007/77961,2007,A2 Location in patent:Page/Page column 271-272
383-63-1
473 suppliers
$10.00/25g

26518-71-8
81 suppliers
$45.00/10mg
![4,4,4-TRIFLUORO-1-(3-OXO-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZIN-6-YL)BUTANE-1,3-DIONE](/CAS/20180601/GIF/943994-23-8.gif)
943994-23-8
16 suppliers
inquiry