
2H-1,4-Benzoxazin-3(4H)-one, 8-Methyl-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)- synthesis
- Product Name:2H-1,4-Benzoxazin-3(4H)-one, 8-Methyl-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
- CAS Number:943994-87-4
- Molecular formula:C15H20BNO4
- Molecular Weight:289.13
![6-BROMO-8-METHYL-4H-BENZO[1,4]OXAZIN-3-ONE](/CAS/GIF/121564-97-4.gif)
121564-97-4
32 suppliers
$327.00/1g

73183-34-3
575 suppliers
$6.00/5g

943994-87-4
29 suppliers
$551.32/250mg
Yield:943994-87-4 98%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90; for 12 h;Inert atmosphere;
Steps:
5.50. 8-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2H-1,4-benzoxazin-3(4H)-one (15b)
A mixture of 4-bromo-2-methyl-6-nitrophenol (9.4 g,40.5 mmol), methyl bromoacetate (6.5 g, 42.5 mmol) and potassium carbonate (8.4 g, 60.8 mmol) in DMSO (50 mL) was stirred at room temperature for 48 h, diluted with water and 10% hydrochloric acid, and extracted with EtOAc. The organic layer was washed with water and brine, dried over Na2SO4 and concentrated in vacuo. The resulting solid was washed with hexane to give methyl (4-bromo-2-methyl-6-nitrophenoxy)acetate (9.86 g, 80%). A mixture of methyl (4-bromo-2-methyl-6-nitrophenoxy)acetate (10.9 g, 35.8 mmol) and zinc dust (35 g, 535 mmol) in acetic acid (100 mL) and THF (200 mL) was stirred at 45 °C for 30 min and at reflux for 1 h, and filtered. The filtrate was concentrated in vacuo,and the residue was diluted with EtOAc. The organic layer was washed with aqueous NaHCO3 solution, water and brine, dried over Na2SO4 and concentrated in vacuo. The residue was was hedwith hexane, suspended in MeOH, and collected by filtration togive 6-bromo-8-methyl-2H-l,4-benzoxazin-3(4H)-one (5.8 g,70%). 1H NMR (300 MHz, DMSO-d6) δ 2.14 (s, 3H), 4.60 (s, 2H),6.87 (d, J = 2.3 Hz, 1H), 7.00 (d, J = 2.3 Hz, 1H), 10.72 (s, 1H). A mixture of 6-bromo-8-methyl-2H-1,4-benzoxazin-3(4H)- one (2.0 g,8.3 mmol), bis(pinacolato)diboron (2.3 g, 9.1 mmol), potassium acetate (2.9 g, 29.5 mmol) [1,1-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane complex (0.34 g,0.42 mmol) in 1,4-dioxane (50 mL) was stirred at 90 °C for 12 hunder argon atmosphere, diluted with water and extracted withEtOAc. The organic layer was dried over Na2SO4 and concentratedin vacuo. The residue was suspended in IPE, and collected by filtration to give 15b (2.36 g, 98%) as a solid. 1H NMR (300 MHz,DMSO-d6) δ 1.27 (s, 12H), 2.15 (s, 3H), 4.61 (s, 2H), 7.06 (s, 1H),7.13 (s, 1H), 10.61 (s, 1H).
References:
Hasui, Tomoaki;Ohyabu, Norio;Ohra, Taiichi;Fuji, Koji;Sugimoto, Takahiro;Fujimoto, Jun;Asano, Kouhei;Oosawa, Masato;Shiotani, Sachiko;Nishigaki, Nobuhiro;Kusumoto, Keiji;Matsui, Hideki;Mizukami, Atsushi;Habuka, Noriyuki;Sogabe, Satoshi;Endo, Satoshi;Ono, Midori;Siedem, Christopher S.;Tang, Tony P.;Gauthier, Cassandra;De Meese, Lisa A.;Boyd, Steven A.;Fukumoto, Shoji [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 19,p. 5428 - 5445]
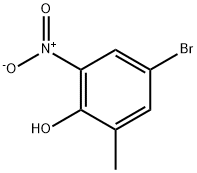
20294-50-2
65 suppliers
$12.00/250mg

943994-87-4
29 suppliers
$551.32/250mg
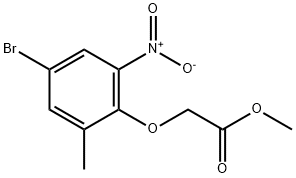
943994-74-9
17 suppliers
inquiry

943994-87-4
29 suppliers
$551.32/250mg