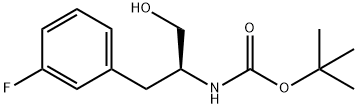
(S)-TERT-BUTYL (1-(3-FLUOROPHENYL)-3-HYDROXYPROPAN-2-YL)CARBAMATE synthesis
- Product Name:(S)-TERT-BUTYL (1-(3-FLUOROPHENYL)-3-HYDROXYPROPAN-2-YL)CARBAMATE
- CAS Number:944470-56-8
- Molecular formula:C14H20FNO3
- Molecular Weight:269.31
114873-01-7
198 suppliers
$6.00/250mg

944470-56-8
25 suppliers
$314.00/1g
Yield:944470-56-8 74%
Reaction Conditions:
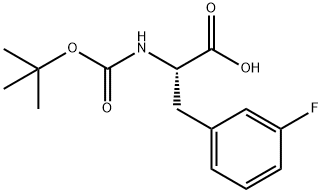
Stage #1: N-Boc-3-fluoro-L-phenylalaninewith borane-THF in tetrahydrofuran at 0; for 12 h;
Stage #2: with methanol;acetic acid in tetrahydrofuran;
Steps:
1.a
To a solution of N-{[(1 ,1-dimethylethyl)oxy]carbonyl}-3-fluoro-L-phenylalanine (10 g, 35.3 mmol) in THF (200 ml.) at 0 0C stirred was added BH3-THF (88 ml_, 88 mmol-1 M in THF). After 12h, the reaction was quenched with AcOH:MeOH (8:50, 58 ml.) and partitioned between saturated aqueous NaHCO3 and DCM. The aqueous phase was then extracted several times with DCM. The combined organic fractions were concentrated and the residue passed through a pad of silica gel (hexanes/EtOAc, 1 :1 ) to afford the product compound (7.0 g, 74%) as a white solid: LCMS (ES) m/e 270 (M+H)+.
References:
WO2009/158371,2009,A1 Location in patent:Page/Page column 33
![Carbamic acid, N-[(1S)-2-(3-fluorophenyl)-1-formylethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/848444-61-1.gif)
848444-61-1
0 suppliers
inquiry

944470-56-8
25 suppliers
$314.00/1g