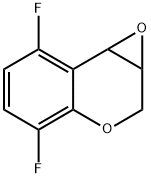
7H-Oxireno[c][1]benzopyran, 2,5-difluoro-1a,7a-dihydro- synthesis
- Product Name:7H-Oxireno[c][1]benzopyran, 2,5-difluoro-1a,7a-dihydro-
- CAS Number:944950-67-8
- Molecular formula:C9H6F2O2
- Molecular Weight:184.14

457628-37-4
16 suppliers
inquiry
![7H-Oxireno[c][1]benzopyran, 2,5-difluoro-1a,7a-dihydro-](/CAS/20211123/GIF/944950-67-8.gif)
944950-67-8
12 suppliers
inquiry
Yield:944950-67-8 60%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 0.5 h;
Steps:
Scheme 15:Step 1 : 5,8-Difluoro-2H-chromene oxide5,8-Difluoro-2H-chromene 61 (35g, 0.21 mol) was dissolved in 500 ml_ of dichloromethane and 93 g of MCPBA (77%, 0.42 mol) was added. The reaction was stirred at room temperature for 30 minutes. The reaction was quenched with a solution of 50 g Na2S2O3 in 500 ml_ water. The organic layer was washed with two 500-mL portions of 2N NaOH solution, 200 ml_ of brine, dried over Na2SO4 and
References:
WO2009/8980,2009,A2 Location in patent:Page/Page column 229-230