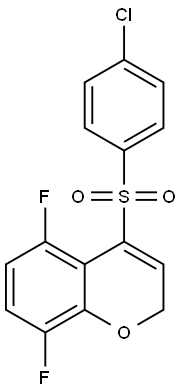
4-(4-chlorophenylsulfonyl)-5,8-difluoro-2H-chroMene synthesis
- Product Name:4-(4-chlorophenylsulfonyl)-5,8-difluoro-2H-chroMene
- CAS Number:944950-71-4
- Molecular formula:C15H9ClF2O3S
- Molecular Weight:342.74
![2H-1-Benzopyran-3-ol, 4-[(4-chlorophenyl)thio]-5,8-difluoro-3,4-dihydro-](/CAS/20210111/GIF/944950-69-0.gif)
944950-69-0
0 suppliers
inquiry

944950-71-4
11 suppliers
$501.56/5MG
Yield:944950-71-4 64%
Reaction Conditions:
Stage #1: 4-(4-chloro-phenylsulfanyl)-5,8-difluoro-chroman-3-olwith 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 3 h;
Stage #2: with methanesulfonyl chloride;triethylamine in dichloromethane at 20; for 1 h;
Steps:
Step 3: 4-(4-Chloro-benzenesulfonyl)-5, 8-difluoro-2H-chromene 4-(4-Chloro-phenylsulfanyl)-5,8-difluoro-chroman-3-ol 63 (23.2 g, 71 mmol) was dissolved in 200 mL of dichloromethane and 31.7 g (77%, 142 mmol) of MCPBA was added. The reaction was stirred at room temperature for 3 hours, then quenched with a solution of 10 g of Na2S2O3 in 50 mL water. The organic layer was washed with 2 100-mL portions of 1 N NaOH solution, 100 mL of brine, dried over Na2SO4 and concentrated. The residue was dissolved in 200 mL of dichloromethane, 16.1 g of mesyl chloride (142 mmol) and 14.3 g of triethylamine (142 mmol) were added. The reaction was stirred at room temperature for one hour. The reaction solution was washed with 100 mL of brine, dried over Na2SO4 and concentrated. The residue was recrystalized from EtOAc/Hexane solution to give 8.4 g pure vinyl sulfone product 64. The residue from mother liquor was purified by column chromatography eluting with a gradient from hexanes to 25% EtOAc/hexane over 55 minute to give an additional 7.1 g vinyl sulfone 64A. Total yield: 15.5 g, 64%. 1H NMR (CDCI3400 MHz δ 7.84 (dd, J
References:
WO2009/8980,2009,A2 Location in patent:Page/Page column 229-231