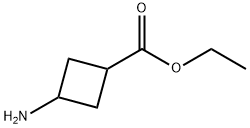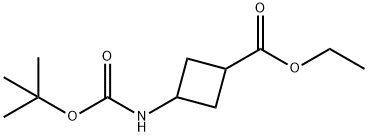
ETHYL 3-((TERT-BUTOXYCARBONYL)AMINO)CYCLOBUTANECARBOXYLATE synthesis
- Product Name:ETHYL 3-((TERT-BUTOXYCARBONYL)AMINO)CYCLOBUTANECARBOXYLATE
- CAS Number:946152-71-2
- Molecular formula:C12H21NO4
- Molecular Weight:243.3
Yield:946152-71-2 90%
Reaction Conditions:
in ethanol at 20; for 24 h;
Steps:
24.1
EXAMPLE 24; 3-Acetylamino-cyclobutanecarboxylic acid [3-[5-(4,6-dimethyl-pyrimidine-5-carbonyl)-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl]-1-(3-fluoro-phenyl)-propyl]-amide (I-39) and 3-acetylamino-cyclobutanecarboxylic acid [3-[5-(4,6-dimethyl-pyrimidine-5-carbonyl)-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl]-1-(3-fluoro-phenyl)-propyl]-amide (I-41); Step 1-; 3-Amino-cyclobutanecarboxylic acid ethyl ester (110a, 0.68 g, 4.75 mmol, CAS Reg No. 74307-73-6) and Boc2O (1.24 g, 5.70 mmol) were dissolved in EtOH (120 mL) with stirring. Stirring was continued for 24 h at RT. The reaction mixture was concentrated and residue purified by flash chromatography eluting with 20% EtOAc/hexanes to afford 1.03 g (90%) of 110b as a colorless liquid which solidifies upon standing: MS m/z=266 (M+Na)+.
References:
US2007/191406,2007,A1 Location in patent:Page/Page column 37
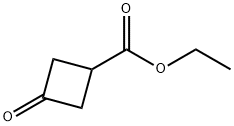
87121-89-9
144 suppliers
$9.00/1g

946152-71-2
5 suppliers
inquiry
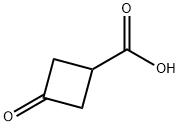
23761-23-1
456 suppliers
$6.00/1g

946152-71-2
5 suppliers
inquiry
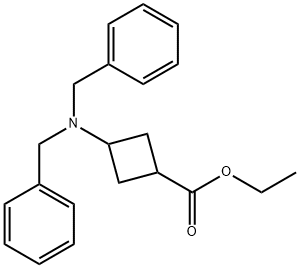
957793-34-9
3 suppliers
inquiry

946152-71-2
5 suppliers
inquiry