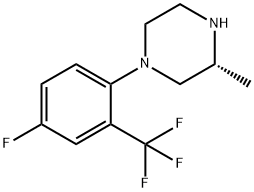
1-(4-Fluoro-2-(trifluoromethyl)phenyl)-3-methylpiperazine synthesis
- Product Name:1-(4-Fluoro-2-(trifluoromethyl)phenyl)-3-methylpiperazine
- CAS Number:946399-84-4
- Molecular formula:C12H14F4N2
- Molecular Weight:262.25
Yield:946399-84-4 98%
Reaction Conditions:
with sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0);2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 105; for 3.5 h;Inert atmosphere;
Steps:
1.1.1A
Step IA: A mixture of (R)-2-methyl-piperazine (25.0 g, 250 mmol), 2-bromo 5- fluoro benzotrifluoride (55.1 g, 227 mmol), tris(dibenzylidineacetone)dipalldium (0) (2.08g, 2.27 mmol), rac-2,2'-bis(diphenylphosphino)-l,r-binaphthyl (4.24 g, 6.81 mmol) and sodium tert-butoxide (27.3 g, 280 mmol) was mixed and purged with N2. Anhydrous toluene (500 mL) was added and purged with N2 again. The resulting mixture was heated in an oil bath at 105 0C under N2 for 3.5 hours. After cooling, the reaction mixture was concentrated and then filtered through a pad of Celite, washed with Et2O. The organic layer was concentrated, diluted with Et2O (500 mL), filtered through a pad of Celite again, and washed with IN aq. HCl (2 x 150 mL). The aqueous layer was basified with NaOH at 0 0C (pH = -10) and then was extracted with Et2O (3 x 200 mL). The combined organic layer was dried over MgSO4 and concentrated under vacuum to give (3i?)-l-[4- fluoro-2-(trifluoromethyl)phenyl]-3-methylpiperazine as a brown oil (58.5 g, 98%), which was used without further purification.
References:
WO2010/141550,2010,A2 Location in patent:Page/Page column 48-49