Yield:946423-58-1 62%
Reaction Conditions:
with hydrogenchloride;water at 110; for 4 h;Product distribution / selectivity;
Steps:
2; 3
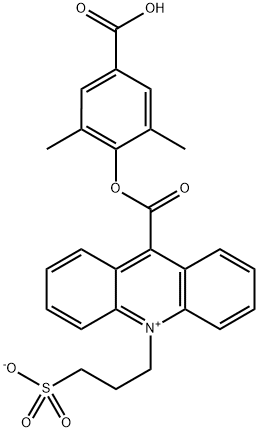
Example 2; [0076] N-Alkyation on a gram scale of 2',6-dimethyl-4'-methoxycarbonylphenyl acridine-9-carboxylate with propane sultone in FBMIMIfPFl: Synthesis of 2',6'- dimethyl-4'-carboxyphenyl-10-sulfopropylacridinium-9-carboxylate.; [0077] To an 8 dram vial was added 2.0 g (5.19 mmol) of the acridine ester, 15 g(52.8 mmol) of l-butyl-3-methylimidazolium hexafluorophosphate (Fluka) and 6.34 g (51.9 mmol) of distilled 1,3-prυpane sultone under nitrogen. This sealed vial was heated to 1550C for 24 hours. Then the reaction mixture was cooled to 4O0C and added drop wise into 500 ml of ethyl acetate with stirring which gave a yellow precipitate. The mixture was stirred at room temperature for 2 hours and filtered. The filter cake was washed with ethyl acetate (50 ml x 3) and dried under high vacuum which gave 6.19 g of a yellow solid which was then treated as follows.[0078] The above 6.19 g of crude acridinium ester was added to 80 ml of HCl solution (8 ml concentrated HCl and 72 ml de-ionized water). The reaction mixture was heated to HO0C for 4 hours and then cooled to 2-80C overnight. A yellow precipitate was formed which was filtered. The filter cake was washed with de-ionized water (40 ml x 3) and diethyl ether (20 ml x 2) and dried under high vacuum over P2O5 which gave 1.68 g yellow powder (66% yield from 2',6'-dimethyl-4'-methoxycarbonylphenyl acridine-9-carboxylate, 94% purity by HPLC).; Example 3; [0079] N-Alkyation on a gram scale of 26-dimethyl-4'-methoxycarbonylphenyl acridine-9-carboxylate with propane sultone in [BMIMl [BF41: Synthesis of 2',6'- dimethyl-4'-carboxyphenyl-10-sulfopropylacridinium-9-carboxylate.; [0080] To an 8 dram vial was added 1.0 g (2.6 mmol) of the acridine ester, 10 g(44.2 mmol) of l-butyl-3-methylimidazolium tetrafluoroborate [BMIM]BF4] (Fluka) and 3.18 g (26.0 mmol) of distilled 1,3-propane sultone under nitrogen. This sealed vial was heated to 1550C for 24 hours. Then the reaction mixture was cooled to room temperature which gave a red, clear liquid (83% conversion to product by HPLC). The above red clear liquid of the acridinium methyl ester was added to a 30 ml of 1 M HCl solution. The reaction mixture was heated to HO0C for 4 hours and then cooled to room temperature which gave a red solution. The reaction mixture was poured into a 1000 ml of de-ionized water and a yellow precipitate was formed. The mixture was kept in the refrigerator overnight. The yellow precipitate was then filtered and washed with de- ionized water (40 ml x 3) and diethyl ether (40 ml x 3). It was then dried under high vacuum over P2O5 which gave 0.793 g of product as a yellow powder (62% yield from acridine ester, 81% purity by HPLC).
References:
WO2009/67417,2009,A1 Location in patent:Page/Page column 26-28
![Acridinium, 9-[[4-(methoxycarbonyl)-2,6-dimethylphenoxy]carbonyl]-10-(3-sulfopropyl)-, inner salt](/CAS/20211123/GIF/946423-59-2.gif)
![9-[(4-Carboxy-2,6-diMethylphenoxy)carbonyl]-10-(3-sulfopropyl)acridiniuM Inner Salt](/CAS/20150408/GIF/946423-58-1.gif)