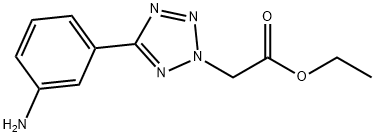
[5-(3-Amino-phenyl)-tetrazol-2-yl]-acetic acid ethyl ester synthesis
- Product Name:[5-(3-Amino-phenyl)-tetrazol-2-yl]-acetic acid ethyl ester
- CAS Number:946817-51-2
- Molecular formula:C11H13N5O2
- Molecular Weight:247.25
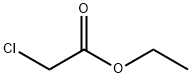
105-39-5
406 suppliers
$10.00/5g
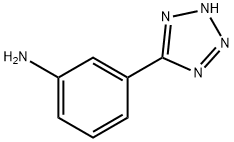
73732-51-1
88 suppliers
$35.35/250mgs:
![[5-(3-Amino-phenyl)-tetrazol-2-yl]-acetic acid ethyl ester](/CAS/20180703/GIF/946817-51-2.gif)
946817-51-2
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 50; for 2 h;
Steps:
12
Ethyl chloroacetate (33 mL, 0.31 mmol), [3-(2H-tetrazol-5-yl)phenyl]amine (50 mg, 0.31 mmol) and K2CO3 (86 mg, 0.62 mmol) were mixed in dry DMF (5 mL). The mixture was heated at 50 °C for 2 h using a Stem block. The reaction mixture was partitioned between water (15 mL) and EtOAc (10 mL). The aqueous layer was extracted once with EtOAc (10 mL). The organic layers were combined, dried (Na2S04), filtered and concentrated to give 60 mg of a light brown gum. The intermediate was dissolved in dry CH2C12 (3 mL) followed by addition of 5-isopropyl-3-methylbenzothiophene-2-sulfonyl chloride (Intermediate 2) (58 mg, 0.20 mmol) and pyridine (113 μΕ, 1.41 mmol). The reaction mixture was stirred for 2 h at room temperature. Aqueous NaOH (1M, 1 mL) was added and the reaction mixture was stirred for an additional 5 minutes. The mixture was acidified with aqueous HC1 (1M, 1 mL) and partitioned between CH2C12 (10 mL) and water (10 mL). The aqueous layer was extracted once with EtOAc (10 mL). The organic layers were combined, dried (Na2S04), filtered and concentrated to give the crude product (250 mg) as a brown gum. The crude product was dissolved in MeOH/DMSO (7: 1, 4 mL) and purified by reversed phase preparative HPLC (ACE C8, 0.1%TFA in water/CH3CN) to give 34 mg of the title compound as a light brown solid (36% over two steps). 1H NMR (400 MHz, MeOU-d4) δ ppm 1.27 (d, 6 H) 2.53 (s, 3 H) 2.95 - 3.07 (m, 1 H) 5.59 (s, 2 H) 7.25 - 7.31 (m, 1 H) 7.33 - 7.40 (m, 2 H) 7.59 - 7.63 (m, 1 H) 7.73 (d, 1 H) 7.79 - 7.85 (m, 1 H) 7.94 - 8.00 (m, 1 H). The structure was confirmed by extended NMR analysis (1H NMR, 13C NMR, gDQFCOSY, 13C-gHSQC, 13C-gHMBC, 15N- gHMBC and DPFGSE-NOE). MS (ESI+) calcd for C2iH2iN504S2 471.103496, found 471.103976.
References:
WO2012/35171,2012,A2 Location in patent:Page/Page column 63