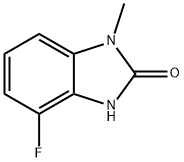
2H-Benzimidazol-2-one, 4-fluoro-1,3-dihydro-1-methyl- synthesis
- Product Name:2H-Benzimidazol-2-one, 4-fluoro-1,3-dihydro-1-methyl-
- CAS Number:947255-04-1
- Molecular formula:C8H7FN2O
- Molecular Weight:166.15
Yield:947255-04-1 78%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1 h;
Steps:
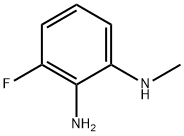
25.3 (Step 3) 4-Fluoro-1-methyl-1H-benzo[d]imidazol-2(3H)-one (P-25)
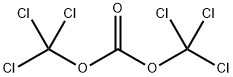
3-Fluoro-N 1-methylbenzene-1,2-diamine (0.18 g, 1.3 mmol) was dissolved in dichloromethane (2 mL), cooled to 0 °C, and triethylamine (0.16 g, 1.6 mmol) was added , slowly add triphosgene/dichloromethane solution (0.15g/1mL) dropwise to the reaction system, and react at room temperature for 1h. The reaction solution was concentrated under reduced pressure, water (20 mL) was added to the concentrate, extracted with dichloromethane three times (10 mL each time), the organic layers were combined, washed once with water and saturated brine, dried over anhydrous sodium sulfate, filtered, It was concentrated under reduced pressure, and the concentrate was purified by silica gel column chromatography (petroleum ether/ethyl acetate=4:1) to obtain the title compound (0.17 g, 78%).
References:
WO2022/156351,2022,A1 Location in patent:Page/Page column 32-33
947255-02-9
5 suppliers
inquiry

947255-04-1
0 suppliers
inquiry

19064-24-5
265 suppliers
$6.00/1g

947255-04-1
0 suppliers
inquiry