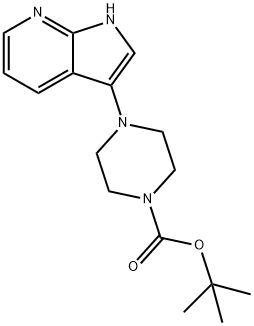
Tert-Butyl 4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidine-1-carboxylate synthesis
- Product Name:Tert-Butyl 4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidine-1-carboxylate
- CAS Number:947498-92-2
- Molecular formula:C16H22N4O2
- Molecular Weight:302.37
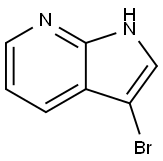
74420-15-8
255 suppliers
$8.00/1g

57260-71-6
736 suppliers
$5.00/5g
![Tert-Butyl 4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidine-1-carboxylate](/CAS2/GIF/947498-92-2.gif)
947498-92-2
16 suppliers
inquiry
Yield:947498-92-2 165 mg
Reaction Conditions:
with chloro-(2-dicyclohexylphosphino-2’,6’-diisopropoxy-1,1‘-biphenyl)[2-(2-aminoethyl)phenyl] palladium(ll) methyl-tert-butyl ether adduct;lithium hexamethyldisilazane;ruphos in tetrahydrofuran;tert-butyl methyl ether at 70; for 3 h;Inert atmosphere;
Steps:
R1.1 Example Ri.Illustrative synthesis of tert-Butyl 4-(1H-pyrrolo j2,3-bJ pyridin-3-yl)piperazine- 1- carboxylate
To a mixture of tert-butyl piperazine-1-carboxylate (224 mg, 1.2 equiv.), 3-bromo-1H-pyrrolo[2,3-b]pyridine (197 mg, 1 equiv.), RuPhos (9.3 mg, 0.02 equiv.) and Ruphos PdG1, MTBE adduct (16.3 mg, 0.02 equiv.) in THF (2 mL), under argon atmosphereLiHIVIDS, 1.3 M THF solution (1.92 mL, 2.5 equiv.) was added. The resulting mixture waspurged with argon for 5 mm, then sealed in vial and heated for 3 h at 70 °C. The reactionmixture was cooled to RT, quenched by the addition of 1M HC1 (1.5 mL), diluted with EtOAc and poured into sodium bicarbonate sat. solution. After extracting with 3 portions of EtOAc, the combined organic layers were dried and solvent evaporated. The obtained residue was purified by flash chromatography on silica gel (eluting with cyclohexane /EtOAc gradient; 0-100 % of EtOAc) to afford the expected product (165 mg). LCMS: MW (calcd): 302.37; MS (ES, m/z): 303.21 [M+H].
References:
WO2018/78360,2018,A1 Location in patent:Page/Page column 144; 145
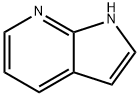
271-63-6
570 suppliers
$9.00/5g
![Tert-Butyl 4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidine-1-carboxylate](/CAS2/GIF/947498-92-2.gif)
947498-92-2
16 suppliers
inquiry