
Tert-Butyl (5-Formylthiazol-2-Yl)(Methyl)Carbamate(WXC00999) synthesis
- Product Name:Tert-Butyl (5-Formylthiazol-2-Yl)(Methyl)Carbamate(WXC00999)
- CAS Number:947602-46-2
- Molecular formula:C10H14N2O3S
- Molecular Weight:242.29
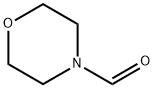
4394-85-8
302 suppliers
$6.00/25g

479198-74-8
18 suppliers
$90.00/100mg

947602-46-2
9 suppliers
inquiry
Yield:947602-46-2 80%
Reaction Conditions:
Stage #1: 2-(N-tert-butoxycarbonyl-N-methyl-amino)-thiazolewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78 - 0;Inert atmosphere;
Stage #2: 4-morpholinecarboxaldehyde in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Steps:
75
To tetrahydrofuran solution (50 ml) of di-isopropylamine (5.12 ml, 36.4 mmol), 1.58 M n-butyllithium/n-hexane solution (23.0 ml, 36.4 mmol) was dropped at -60°C or below in argon atmosphere, and then, the mixture was slowly warmed up to 0°C. Then, tetrahydrofuran solution (10 ml) of 48 (7.08 g, 33.1 mmol) was dropped at -70°C or below, and the mixture was stirred at -78°C for 1 hr. Then, tetrahydrofuran solution (10 ml) of N-formylmorpholine (4.98 ml, 49.6 mmol) was added all at once at the same temperature, and the mixture was stirred for 30 min at the same temperature. The reaction mixture was added cold water, and extracted with ethyl acetate. The extract was washed with water, dried, and evaporated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography (solvent: n-hexane/ethyl acetate = 4/1), to obtain 49 (6.40 g, 80%) of yellow solid substance. mp 80~81° C, 1H NMR(500MHz, DMSO-D6)δ 1.55(9H,s),3.52(3H,s),8.38(1H,s),9.90(1H,s) IR(Nujol) 1714, 1659cm-1, APCI-MS m/z 243[M+H]+
References:
EP2103611,2009,A1 Location in patent:Page/Page column 60-61