
6-BROMO-2-METHYLQUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:6-BROMO-2-METHYLQUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:948289-14-3
- Molecular formula:C13H12BrNO2
- Molecular Weight:294.14
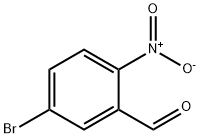
20357-20-4
143 suppliers
$5.00/250mg
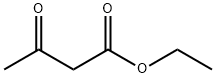
141-97-9
781 suppliers
$5.00/25g

948289-14-3
29 suppliers
$308.00/1g
Yield:948289-14-3 55%
Reaction Conditions:
Stage #1: 5-bromo-2-nitrobenzaldehyde;ethyl acetoacetatewith tin(ll) chloride;zinc(II) chloride in diethyl ether;ethanol at 70; for 3 h;Molecular sieve;
Stage #2: with water;sodium hydrogencarbonate in diethyl ether;ethanol at 20;
Steps:
35.35a
35a) Ethyl 6-bromo-2-methyl-3-quinolinecarboxylate A 2.2 M solution of zinc(II)chloride (9.88 mL, 21.74 mmol) in diethyl ether was added to tin(II)chloride (4.12 g, 21.74 mmol) and activated 4 ? molecular sieve pellets (1.00 g) in ethanol (10 mL) under argon. Then, 5-bromo-2-nitro-benzaldehyde (1.00 g, 4.35 mmol) and ethyl 3-oxobutanoate (594.0 mg, 4.56 mmol) in ethanol (12 mL) were added to the reaction mixture via canula. The mixture was heated at 70° C. in an oil bath for three hours, then allowed to cool to room temperature, and carefully quenched with saturated sodium bicarbonate. Ethyl acetate was added and the mixture was filtered through Celite. The filtrate was extracted with ethyl acetate and the organic layer was dried over anhydrous magnesium sulfate, then filtered and concentrated. The residue was purified by silica gel chromatography eluding with 1:6 ethyl acetate:hexanes to give 697.9 mg (55%) of ethyl 6-bromo-2-methyl-3-quinolinecarboxylate as a solid. 1H NMR (400 MHz, d6-DMSO): δ 8.84 (s, 1H), 8.42 (d, J=2 Hz, 1H), 7.95 (dd, J=9, 2 Hz, 1H), 7.91 (d, J=9 Hz, 1H), 4.38 (q, J=7 Hz, 2H), 2.84 (s, 3H), 1.37 (t, J=7 Hz, 3H). ESI-LCMS m/z 294 (M+H)+.
References:
US2008/96921,2008,A1 Location in patent:Page/Page column 85-86
5794-88-7
464 suppliers
$6.00/10g

948289-14-3
29 suppliers
$308.00/1g

20712-12-3
117 suppliers
$10.00/250mg

948289-14-3
29 suppliers
$308.00/1g
29124-57-0
170 suppliers
$16.00/250mg

141-97-9
781 suppliers
$5.00/25g

948289-14-3
29 suppliers
$308.00/1g