7-[2-(4-Morpholinyl)ethoxy]-2-(4-nitrophenyl)imidazo[2,1-b]benzothiazole synthesis
- Product Name:7-[2-(4-Morpholinyl)ethoxy]-2-(4-nitrophenyl)imidazo[2,1-b]benzothiazole
- CAS Number:950769-60-5
- Molecular formula:C21H20N4O4S
- Molecular Weight:424.47
Yield:950769-60-5 96%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 90 - 95; for 5 h;
Steps:
4.C
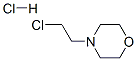
1. A 50 L 3 -neck round bottom flask was equipped with a mechanical agitator, thermocouple probe, drying tube, reflux condenser, and a heating mantle.2. The flask was charged with 2-(4-nitrophenyl) imidazo [2,1- 6]benzothiazol-7-ol (1.770 Kg), N,N-dimethylformamide (18.0 L), 4-(2- chloroethyl)morpholine hydrochloride (2.751 Kg), potassium carbonate (2.360 Kg) and tetrabutylammonium iodide (0.130 Kg) with stirring.3. The resulting yellow suspension was heated to 900C to 95 0C, maintaining the temperature for approximately 5 hours.4. The reaction was monitored by TLC until no starting material was observed. (20% methanol/ethyl acetate; Rf (product) = 0.15; Rf (starting material) = 0.85).5. The reaction mixture was cooled to ~10°C, the yellow solids collected by filtration onto a polypropylene filter pad. The solids were slurried in water (2 x 5 L) and filtered.6. The crude wet product was slurried in acetone (5 L), filtered and the solids rinsed with acetone (2 x 1.5 L).7. The solids were dried in a vacuum oven (<10mmHg) at 45°C. Yield: 2.300 Kg (95%), yellow solid.TLC: R/= 0.15 (20% methanol/EtOAc)HPLC: 95.7% (area)1H NMR: conforms (300 MHz, DMSO) Table 5.
References:
WO2010/54058,2010,A1 Location in patent:Page/Page column 76-78
![2-(4-Nitrophenyl)imidazo[2,1-b]benzothiazol-7-ol](/CAS2/GIF/914224-34-3.gif)