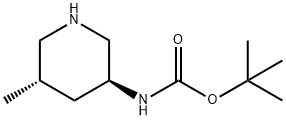
Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester
- CAS Number:951163-61-4
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3

951163-63-6
16 suppliers
inquiry
![Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/GIF/951163-61-4.gif)
951163-61-4
78 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium on activated carbon in ethanol at 45; under 2327.23 Torr;
Steps:
1.A
(3S,5S)-(5-Methyl-piperidin-3-yl)-carbamic acid tert-butyl ester (8). A 40 L pressure vessel is charged with 0.6 Kg 50% wet, solid palladium on carbon (E101, 10 wt. %) under flow of nitrogen. A solution of 3.2 Kg intermediate (7) in 13.7 Kg of absolute ethanol is then charged to the reactor under nitrogen. The reactor is purged with nitrogen and is then pressurized with hydrogen at 45 psi. The reaction is then heated to 45° C. while maintaining a hydrogen pressure of 45 psi. The reaction is monitored by TLC or LC until complete. The reaction is cooled to ambient temperature, vented, and purged with nitrogen. The reactor contents are filtered through a bed of Celite and the solids are washed with 2.8 Kg of absolute ethanol. The filtrate is concentrated by rotary evaporation under vacuum until a waxy solid is obtained to afford intermediate (8): TLC Rf (Silica F254, 70:30 v/v ethyl acetate-hexanes, KMnO4 stain)=0.12; 1H NMR (300 MHz, CDCl3) δ 5.31 (br s, 1H), 3.80-3.68 (m, 1H), 2.92 (d, J=11.4 Hz, 1H), 2.77 (AB quart, JAB=12.0 Hz, Δν=50.2 Hz, 2H), 2.19 (t, J=10.7 Hz, 1H), 1.82-1.68 (m, 2H), 1.54 (br s, 1H), 1.43 (s, 9H), 1.25-1.15 (m, 1H), 0.83 (d, J=6.6 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 155.3, 78.9, 54.3, 50.8, 45.3, 37.9, 28.4, 27.1, 19.2; MS (ESI+) m/z 215 (M+H), 429 (2M+H).
References:
US2007/232650,2007,A1 Location in patent:Page/Page column 6
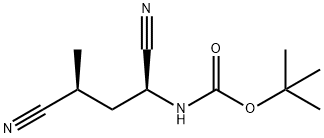
1229616-47-0
3 suppliers
inquiry
![Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/GIF/951163-61-4.gif)
951163-61-4
78 suppliers
inquiry
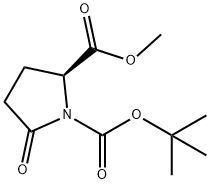
108963-96-8
292 suppliers
$5.00/1g
![Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/GIF/951163-61-4.gif)
951163-61-4
78 suppliers
inquiry

24424-99-5
858 suppliers
$13.50/25G
![Carbamic acid, N-[(3S,5S)-5-methyl-3-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/GIF/951163-61-4.gif)
951163-61-4
78 suppliers
inquiry