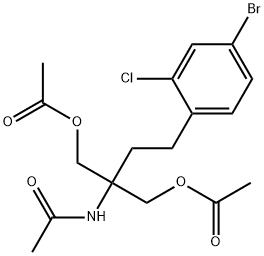
N-[1,1-Bis[(acetyloxy)methyl]-3-(4-bromo-2-chlorophenyl)propyl]acetamide synthesis
- Product Name:N-[1,1-Bis[(acetyloxy)methyl]-3-(4-bromo-2-chlorophenyl)propyl]acetamide
- CAS Number:951238-23-6
- Molecular formula:C17H21BrClNO5
- Molecular Weight:434.71
![Carbamic acid, N-[3-(4-bromo-2-chlorophenyl)-1,1-bis(hydroxymethyl)propyl]-, 1,1-dimethylethyl ester](/CAS/20200515/GIF/916516-93-3.gif)
916516-93-3
0 suppliers
inquiry

108-24-7
5 suppliers
$14.00/250ML
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-bromo-2-chlorophenyl)propyl]acetamide](/CAS/GIF/951238-23-6.gif)
951238-23-6
9 suppliers
$165.00/2mg
Yield:-
Reaction Conditions:
Stage #1: [1,1-bis(hydroxymethyl)-3-(4-bromo-2-chlorophenyl)propyl]carbamic acid tert-butyl esterwith hydrogenchloride in water;ethyl acetate at 30; for 1 h;
Stage #2: with sodium hydroxide in water;
Stage #3: acetic anhydridewith pyridine at 20; for 11 h;
Steps:
21 N-[1,1-bis(acetoxymethyl)-3-(4-bromo-2-chlorophenyl)propyl]acetamide
Reference Example 21 N-[1,1-bis(acetoxymethyl)-3-(4-bromo-2-chlorophenyl)propyl]acetamide [1,1-Bis(hydroxymethyl)-3-(4-bromo-2-chlorophenyl)propyl]carbamic acid tert-butyl ester (175 g) of Reference Example 20 was dissolved in 4M-HCl/ethyl acetate (326 mL), and the mixture was stirred at 30°C for 1 hr. The mixture was concentrated under reduced pressure, and 0.1M aqueous sodium hydroxide solution (2713 mL) was added to the residue. After partitioning and extraction with ethyl acetate, the extract was washed with 25% brine, and dried over magnesium sulfate. The solvent was evaporated under reduced pressure, the residue was dissolved in pyridine (271 mL), and acetic anhydride (270 mL) was added under ice-cooling. After stirring at room temperature for 11 hr, ethyl acetate (2710 mL), 1M aqueous sodium hydroxide solution (1084 mL) and 9% aqueous sodium hydrogen carbonate solution (5420 mL) were successively added to the reaction mixture. After partitioning and extraction with ethyl acetate, the extract was washed with 1M hydrochloric acid, 0.1M hydrochloric acid, 9% aqueous sodium hydrogen carbonate solution and 25% brine, and dried over sodium sulfate. The solvent was evaporated under reduced pressure, and the residual pale-yellow crystals were suspended in a mixture of isopropyl ether (880 mL) and n-heptane (880 mL), and collected by filtration to give the title compound (135.3 g) as white crystals. 1H-NMR(CDCl3)δ(ppm):2.00(3H, s), 2.10(6H, s), 2.12-2.16(2H, m), 2.66-2.72(2H, m), 4.36(4H, s), 5.75(1H, s), 7.10(1H, d, J=8.2Hz), 7.32(1H, dd, J=1.9, 8.2Hz), 7.50(1H, d, J=1.9Hz).
References:
EP2017257,2009,A1 Location in patent:Page/Page column 24