
(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate synthesis
- Product Name:(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate
- CAS Number:951694-73-8
- Molecular formula:C27H46O4
- Molecular Weight:434.65

462122-38-9
24 suppliers
inquiry
![(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate](/CAS/20180703/GIF/951694-73-8.gif)
951694-73-8
15 suppliers
inquiry
Yield:951694-73-8 93%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 45; for 12 h;
Steps:
5 Example 5: Synthesis of Compound 6
The compound 5 (15 g, 1 eq) obtained in the previous step was dissolved in ethanol (150 mL), and then warmed to 45°, and sodiumborohydride (1.6 g, 1.2 eq) wasadded portionwise, and the reaction was kept for 12 h.After the reaction was completed, it was cooled to room temperature, and the reactionsystem wasquenched with 100 mL of saturated sodium hydrogen carbonate.The reaction solution was extracted with 100 mL of dichloromethane, and the organic layer was concentrated to dryness to afford Compound 6 (14 g, yield 93%).
References:
CN107936078,2018,A Location in patent:Paragraph 0041-0043
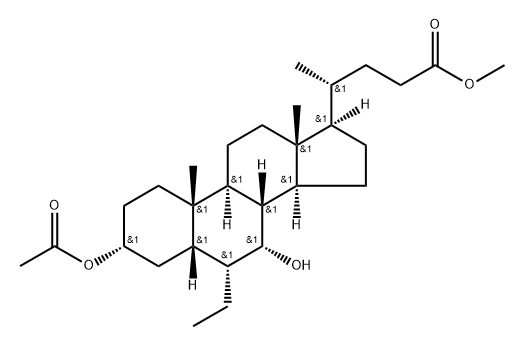
1227612-51-2
1 suppliers
inquiry
![(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate](/CAS/20180703/GIF/951694-73-8.gif)
951694-73-8
15 suppliers
inquiry
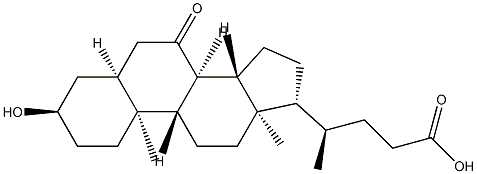
4651-67-6
326 suppliers
$39.00/500mg
![(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate](/CAS/20180703/GIF/951694-73-8.gif)
951694-73-8
15 suppliers
inquiry
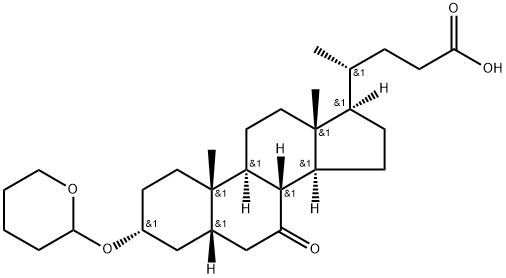
122960-85-4
5 suppliers
inquiry
![(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate](/CAS/20180703/GIF/951694-73-8.gif)
951694-73-8
15 suppliers
inquiry

863239-59-2
75 suppliers
inquiry
![(R)-methyl 4-((3R,5S,6R,7R,10S,13R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate](/CAS/20180703/GIF/951694-73-8.gif)
951694-73-8
15 suppliers
inquiry