
ETHYL 2-AMINO-4-METHYL-4,5,6,7-TETRAHYDRO-1-BENZOTHIOPHENE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 2-AMINO-4-METHYL-4,5,6,7-TETRAHYDRO-1-BENZOTHIOPHENE-3-CARBOXYLATE
- CAS Number:95211-67-9
- Molecular formula:C12H17NO2S
- Molecular Weight:239.33
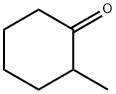
583-60-8
169 suppliers
$10.00/1g
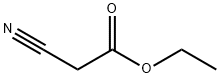
105-56-6
431 suppliers
$10.00/5ml

95211-67-9
28 suppliers
$357.00/1g
Yield:95211-67-9 80.9%
Reaction Conditions:
with morpholine;sulfur in ethanol at 20 - 45; for 29 h;Gewald reaction;
Steps:
6.1. Ethyl 2-amino-4-methyl-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate (9)
A mixture of sulfur (1.1 g, 36 mmol), 2-methylcyclohexanone (4.04 g, 36 mmol), ethyl cyanoactetate (4.07 g, 36 mmol) and EtOH (150 mL) were placed in a round bottom flask and warmed to 45 °C and treated dropwise with morpholine (3.1 g, 36 mmol) over 15 min. The mixture was stirred for 5 h at 45 °C and 24 h at room temperature. Unreacted sulfur was removed by filtration, and the filtrate was concentrated under reduced pressure to afford an orange solid. The residue was loaded on a silica gel column packed with silica gel and eluted with 10% ethyl acetate in hexane. The fractions containing the desired product (TLC) were pooled and evaporated to afford 9 (6.97 g, 80.9%) as an orange solid; mp 69.9-71 °C; Rf 0.44 (hexane/EtOAc 3:1); 1H NMR (DMSO-d6): δ 1.08-1.10 (d, 3H, J = 6.6 Hz, 4-CH3), 1.25-1.28 (t, 3H, J = 6.8 Hz, COOCH2CH3), 1.57-1.78 (m, 4H), 2.39-2.43 (m, 2H), 3.15-3.17 (m, 1H), 4.10-4.24 (q, 2H, J = 6.8 Hz, COOCH2CH3), 7.23 (s, 2H, NH2 exch). Titled compound was used directly for next step without further characterization.
References:
Zhang, Xin;Zhou, Xilin;Kisliuk, Roy L.;Piraino, Jennifer;Cody, Vivian;Gangjee, Aleem [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 11,p. 3585 - 3594] Location in patent:experimental part
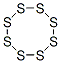
10544-50-0
22 suppliers
inquiry

583-60-8
169 suppliers
$10.00/1g

105-56-6
431 suppliers
$10.00/5ml

95211-67-9
28 suppliers
$357.00/1g