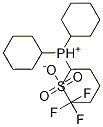
Tricyclohexylphosphonium trifluoromethanesulfonate, 99% Stabiphos synthesis
- Product Name:Tricyclohexylphosphonium trifluoromethanesulfonate, 99% Stabiphos
- CAS Number:952649-12-6
- Molecular formula:[(C6H11)3PH]+CF3SO3-
- Molecular Weight:431
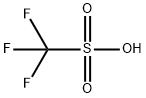
1493-13-6
608 suppliers
$12.00/5g
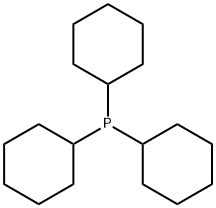
2622-14-2
391 suppliers
$6.00/1g

952649-12-6
14 suppliers
$57.00/1g
Yield:952649-12-6 83%
Reaction Conditions:
in tert-butyl methyl ether;Product distribution / selectivity;
Steps:
2
Example 2: Preparation of tricyclohexyl phosphonium trifluoromethane sulfonate from the tricyclohexyl phosphine carbon disulfide adduct: 80 ml of a solution of 116.7 g cyclohexylchloride in 390 ml of diethylether and 0.5 ml of 1.2-dibromoethane are added to 23.9 g of magnesium turnings, while the reaction mixture is heated to 41 °C. The remaining cyclohexylchloride solution is slowly added to the mixture under reflux. After two hours of stirring at reflux the Grignard solution is diluted with 60 ml of tert-butylmethylether, added to a solution of 45.0 g of phosphorus trichloride in 170 ml tert-butylmethylether at -17 to -7°C within 140 min, and stirred over night at room temperature. After one hour of stirring at reflux the mixture is added to 220 ml of 15% ammonium chloride solution at 10 to 38°C, and the paste-like lower phase is diluted with 140 ml of water. The lower aqueous phase is discarded, and to the upper organic phase are added 25.0 g of carbon disulfide. The resulting precipitated reddish-brown solid is filtered off, washed three times each with 50 ml of petrol ether, and dried at 35°C under vacuum. 72.7 g (62%) of tricyclohexyl phosphine carbon disulfide adduct is obtained. 17.8 g of this tricyclohexyl phosphine carbon disulfide adduct is added to 100 ml of ethanol, and 96 ml of this mixture is distilled off. To the slightly opaque, yellowish melt is again added 100 ml of ethanol, and an additional 97 ml of solvent are distilled off. This process is repeated with 50 ml of ethanol and 49 ml of distillate. The tricyclohexyl phosphine released from the carbon disulfide in residue is taken up in 100 ml tert-butylmethylether, and added to 4,4 ml trifluoromethane sulfonic acid and additional 50 ml tert-butylmethylether for the dilution of the product suspension. The solid is filtered off, and dried at 40°C. 17.8 g (83%) colorless tricyclohexyl phosphonium trifluoromethane sulfonate is obtained.
References:
EP2019107,2009,A1 Location in patent:Page/Page column 6