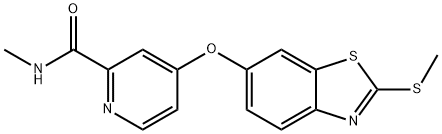
N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide synthesis
- Product Name:N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide
- CAS Number:953770-85-9
- Molecular formula:C15H13N3O2S2
- Molecular Weight:331.41

74537-49-8
38 suppliers
$33.00/100mg
220000-87-3
481 suppliers
$8.00/5g
![N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide](/CAS/20150408/GIF/953770-85-9.gif)
953770-85-9
25 suppliers
$57.00/100mg
Yield:953770-85-9 62%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20 - 70;Heating / reflux;
Steps:
15.3 Step 3.
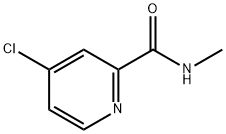
Step 3. Preparation of 4-(2-Methylsulfanyl-benzothiazol-6-yloxy)-pyridine-2-carboxylic acid methylamide To the solution of 2-methylsulfanyl-benzothiazol-6-ol (3.76 g, 19.08 mmol, 1.0 eq) in DMF (25 mL), was added CsCO3 (15.54 g, 47.70 mmol, 2.5 eq) at room temperature. After stirring for a while, 4-chloro-pyridine-2-carboxylic acid methylamide (4.86 g, 28.62 mmol, 1.5 eq) was added to the mixture and the mixture was stirred at 70° C. under reflux condenser overnight. After cooling the reaction mixture in ice bath, water (100 mL) was added and the aqueous layer was extracted with ethyl acetate (3*150 mL). The organic layer was dried over sodium sulfate, filtered, and evaporated in vacuo. The crude product was purified using 20 g of ISCO Silica Gel column (0%-50%-80%-100% ethyl acetate-hexane mixture over 45 min 40 mL/min run) to yield 4-(2-methylsulfanyl-benzothiazol-6-yloxy)-pyridine-2-carboxylic acid methylamide (3.88 g, 62%) as a white solid. M+H=332.1
References:
US2008/45528,2008,A1 Location in patent:Page/Page column 47