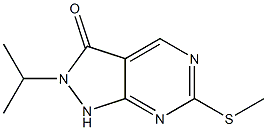
2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one synthesis
- Product Name:2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one
- CAS Number:955368-93-1
- Molecular formula:C9H12N4OS
- Molecular Weight:224.2828
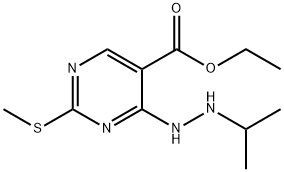
955368-92-0
2 suppliers
inquiry
![2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one](/CAS/20200401/GIF/955368-93-1.gif)
955368-93-1
7 suppliers
inquiry
Yield:955368-93-1 74.7%
Reaction Conditions:
Stage #1: ethyl 2-(methylsulfanyl)-4-[2-(propan-2-yl)hydrazin-1-yl]pyrimidine-5-carboxylatewith water;sodium hydroxide in methanol at 0; for 2 h;
Stage #2: with hydrogenchloride in methanol;water at 0 - 20; pH=Ca. 2; for 3 h;
Steps:
S-1.3 Step-3: Synthesis of 2-isopropyl-6-methylsulfanyl-1H-pyrazolo[3,4- d]pyrimidin-3-one:
In a single neck 2L RBF, ethyl 4-(2-isopropylhydrazino)-2- methylsulfanyl-pyrimidine-5-carboxylate (18.0 g, 66.63 mmol, 1.0 eq) was dissolved in methanol (200 mL) and solution was cooled to 0 °C. A solution of sodium hydroxide in water (5N, 600 mL) was added drop wise to reaction solution and reaction mixture was stirred at same temperature for 2 hour. Hydrochloric acid in water (5N, 500 mL) was added drop wise to make pH ~ 2 and stirred for 3 hours at ambient temperature. After completion of reaction, dichloromethane (1 L x 2) was added to reaction mixture and organic phase was extracted. Organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. Solid obtained was washed with pentane (100 mL x 2) to afford 11.17 g (74.7%) of 2-isopropyl-6-methylsulfanyl-1H-pyrazolo [3,4-d]pyrimidin-3-one as pale solid. LCMS: 225.2 [M+1]+. 1H NMR (400 MHz, Chloroform-*): d 8.72 (s, 1H), 4.82 (h, J = 6.7 Hz, 1H), 2.59 (d, J = 10.0 Hz, 3H), 1.41 (d, J = 6.7 Hz, 6H).
References:
WO2020/210380,2020,A1 Location in patent:Paragraph 0135; 0137
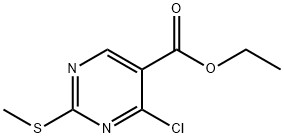
5909-24-0
404 suppliers
$6.00/5g
![2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one](/CAS/20200401/GIF/955368-93-1.gif)
955368-93-1
7 suppliers
inquiry

117147-06-5
7 suppliers
inquiry
![2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one](/CAS/20200401/GIF/955368-93-1.gif)
955368-93-1
7 suppliers
inquiry
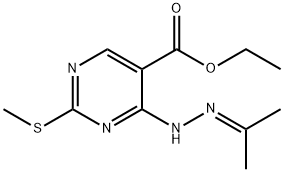
109962-43-8
2 suppliers
inquiry
![2-isopropyl-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one](/CAS/20200401/GIF/955368-93-1.gif)
955368-93-1
7 suppliers
inquiry