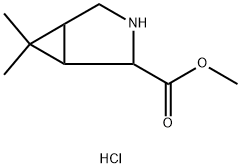
(1S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate synthesis
- Product Name:(1S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate
- CAS Number:956004-47-0
- Molecular formula:C9H16ClNO2
- Molecular Weight:205.68
![3-Azabicyclo[3.1.0]hexane-2,3-dicarboxylic acid, 6,6-dimethyl-, 3-(1,1-dimethylethyl) 2-methyl ester](/CAS/20180703/GIF/956004-48-1.gif)
956004-48-1
0 suppliers
inquiry
![(1S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/GIF/956004-47-0.gif)
956004-47-0
13 suppliers
inquiry
Yield:956004-47-0 44.9%
Reaction Conditions:
with hydrogenchloride in ethyl acetate at 25;
Steps:
21
Example 21; Synthesis Example of methyl 6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride 0.32 g (1.2 mmol) of methyl 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate, and 1.16 g of an ethyl acetate solution containing 15 wt% of hydrogen chloride were mixed at 25°C, then, thermally insulated at 25°C. Disappearance of methyl 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate was confirmed, then, the reaction liquid was concentrated under reduced pressure to distill off the solvent. To the resultant concentration residue was added 0.32 g of toluene and 0.5 ml of methanol, and the mixture was heated up to 40°C to dissolve the deposited crystal. Into the resultant solution, 1.6 ml of methyl tert-butyl ether was dropped. A slurry containing the deposited crystal was cooled down to 0°C. The slurry liquid was filtrated to obtain a crystal which was then washed using methyl tert-butyl ether. The resultant crystal was dried under reduced pressure, to obtain 0.11 g (0.53 mmol) of methyl 6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride. The yield of methyl 6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride was 44.9% with respect to methyl 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate.
References:
EP2014648,2009,A1 Location in patent:Page/Page column 22
![3-Azabicyclo[3.1.0]hexane-3-carboxylic acid, 2-cyano-6,6-dimethyl-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/956004-44-7.gif)
956004-44-7
0 suppliers
inquiry
![(1S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate](/CAS/GIF/956004-47-0.gif)
956004-47-0
13 suppliers
inquiry