
2-(4-Methoxyphenyl)-4H-furo[3,2-b]pyrrole-5-carboxylic Acid Ethyl Ester synthesis
- Product Name:2-(4-Methoxyphenyl)-4H-furo[3,2-b]pyrrole-5-carboxylic Acid Ethyl Ester
- CAS Number:956296-80-3
- Molecular formula:C16H15NO4
- Molecular Weight:285.29
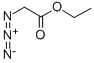
637-81-0
164 suppliers
$40.00/500mg
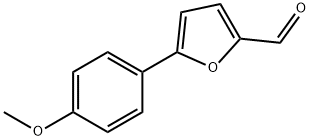
34070-33-2
46 suppliers
$235.00/1g
![2-(4-Methoxyphenyl)-4H-furo[3,2-b]pyrrole-5-carboxylic Acid Ethyl Ester](/CAS/20150408/GIF/956296-80-3.gif)
956296-80-3
3 suppliers
inquiry
Yield:956296-80-3 2.32 g
Reaction Conditions:
with sodium ethanolate in ethanol;toluene at 0; for 3.5 h;Reflux;
Steps:
1 Preparation Example 1
Synthesis of Near Infrared Fluorescent Material A
Next, under an argon stream, the compound (a-1) (3.39 g, 16.8 mmol) and ethyl azidoacetate (8.65 g, 67.0 mmol) were dissolved in ethanol (300 mL) in a 1 L three-neck flask, and a 20% by mass sodium ethoxide ethanol solution (22.8 g, 67.0 mmol) was slowly added dropwise to the obtained solution at 0° C. in an ice bath, followed by stirring for 2 hours.
After the reaction ended, a saturated ammonium chloride aqueous solution was added thereto to adjust the pH to be weakly acidic, water was added thereto, suction filtration was performed, and the obtained material was dried, whereby ethyl 2-azido-3-[5-(4-methoxyphenyl)-furan-2-yl] acrylate (a-2) was obtained as a yellow solid (obtained amount: 3.31 g, yield: 63.1%).
Furthermore, the compound (a-2) (3.31 g, 10.6 mmol) was put into a 200 mL egg-plant shaped flask, and this was dissolved in toluene (60 mL), followed by refluxing and stirring for 1.5 hours. After the solution after refluxing and stirring was concentration under reduced pressure, the obtained crude product was recrystallized (solution: hexane and ethyl acetate), then, the resultant product was subjected to suction filtration, and the obtained material was dried, whereby 2-(4-methoxyphenyl)-4H-furo[3.2-b]pyrrole-5-carboxylic acid ethyl ester (a-3) was obtained as a brown crystal (obtained amount: 2.32 g, yield: 76.8%).
References:
US2016/271273,2016,A1 Location in patent:Paragraph 0258-00259
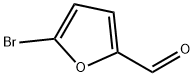
1899-24-7
265 suppliers
$13.43/500mgs:
![2-(4-Methoxyphenyl)-4H-furo[3,2-b]pyrrole-5-carboxylic Acid Ethyl Ester](/CAS/20150408/GIF/956296-80-3.gif)
956296-80-3
3 suppliers
inquiry
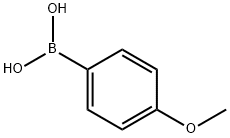
5720-07-0
455 suppliers
$6.00/5g
![2-(4-Methoxyphenyl)-4H-furo[3,2-b]pyrrole-5-carboxylic Acid Ethyl Ester](/CAS/20150408/GIF/956296-80-3.gif)
956296-80-3
3 suppliers
inquiry