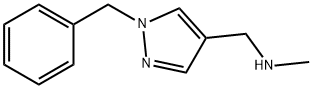
1H-Pyrazole-4-methanamine, N-methyl-1-(phenylmethyl)- synthesis
- Product Name:1H-Pyrazole-4-methanamine, N-methyl-1-(phenylmethyl)-
- CAS Number:956438-25-8
- Molecular formula:C12H15N3
- Molecular Weight:201.27
Yield:956438-25-8 125 mg
Reaction Conditions:
Stage #1: 1-benzyl-1H-pyrrole-3-carbaldehyde;methylamine hydrochloridewith triethylamine in 1,2-dichloro-ethane at 20; for 4 h;
Stage #2: with sodium tetrahydroborate in 1,2-dichloro-ethane;
Steps:
138.1
Example 1383- r3-((S)-1-Amino-propyl)-6-chloro-2-fluoro-phenoxyl-N-n-benzyl-1 H-pyrazol-4-ylmethyl)-N- methyl-benzamide. hydrochlorideStep 1 Triethylamine (0.45 ml, 3.2 mmol) was added to a solution of 1-benzyl-4- formyl pyrrole (300 mg, 1.6 mmol) and methylamine hydrochloride (217 mg, 3.2 mmol) in DCE (6 ml). The resulting solution was stirred for 4 hours at room temperature before sodium borohydride (122 mg, 3.2 mmol) was added and the reaction stirred overnight. The mixture was partitioned between sat. ammonium chloride and DCM and the combined organic fractions were dried over sodium sulfate, filtered and evaporated. The residue was purified by preparative hplc to afford (1-benzyl-1 H-pyrazol-4-ylmethyl)-methyl-amine (125 mg) as a colourless oil. MS: [M+H]+ 202.
References:
WO2013/64538,2013,A1 Location in patent:Page/Page column 159

