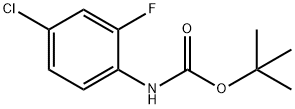
(4-chloro-2-fluoro-phenyl)-carbaMic acid tert-butyl ester synthesis
- Product Name:(4-chloro-2-fluoro-phenyl)-carbaMic acid tert-butyl ester
- CAS Number:956828-47-0
- Molecular formula:C11H13ClFNO2
- Molecular Weight:245.68

24424-99-5

956828-47-0
The general procedure for the synthesis of tert-butyl (4-chloro-2-fluorophenyl)-carbamate from di-tert-butyl dicarbonate is as follows: Example 1: N-(4-chloro-2-fluorophenyl)-N-methyl-3-oxo-1H,3H-spiro[2-benzofuran-1,3'-pyrrolidine]-1'-carboxamide Step 1. Synthesis of tert-butyl tert-butyl (4-chloro-2-fluorophenyl)carbamate To a solution of 4-chloro-2-fluoroaniline (4.0 mL, 0.036 mol; Aldrich) in tetrahydrofuran (40 mL, 0.4 mol) was added slowly and dropwise 1.0 M lithium hexamethyldisilazide in tetrahydrofuran (72 mL) at 0 °C for 1 hour. After the reaction solution turned bright purple, it was warmed to room temperature and stirring was continued for 30 min. Subsequently, a tetrahydrofuran solution (20 mL, 0.2 mol) of di-tert-butyl dicarbonate (8.30 g, 0.0380 mol) was added dropwise over 10 minutes. The reaction mixture was stirred at room temperature for 35 min before the reaction was quenched with saturated ammonium chloride solution and diluted with ethyl acetate to separate the organic layer. The combined organic layers were dried with anhydrous sodium sulfate and concentrated to dryness under reduced pressure. The residue was purified by combiflash fast column chromatography using a gradient elution with a hexane solution of 0-10% ethyl acetate to give 4.33 g of orange solid product in 48.9% yield.The LCMS assay showed [M + H]+ of 246.1.

24424-99-5
861 suppliers
$13.50/25G
57946-56-2
355 suppliers
$12.00/25g

956828-47-0
22 suppliers
$12.00/250mg
Yield:956828-47-0 89%
Reaction Conditions:
in 1,4-dioxane; for 48 h;Reflux;
Steps:
17.1 Step-1: tert-Butyl(4-chloro-2-fluorophenyl)carbamate
A solution of 4-chloro-2-fluoroaniline (2 g, 13.74 mmol) and di-tert-butyl dicarbonate (6.4 mL, 27.6 mmol) in 1,4-dioxane (50 mL) was stirred at reflux for 2 days.
The solvent was then evaporated.
The resulting oil was diluted with MeOH, water, and aqueous ammonium hydroxide solution (10 mL each) and vigorously stirred for 45 minutes.
The organic lower layer was separated.
The organic material was diluted with EtOAc (50 mL), and washed with water (50 mL), 3.6% aqueous HCl solution (2*50 mL), saturated aqueous NaHCO3 solution (50 mL), and then again with water (2*50 mL).
The organic layer was dried (MgSO4), filtered, and evaporated under reduced pressure to provide tert-butyl(4-chloro-2-fluorophenyl)carbamate (3.0011 g, 12.22 mmol, 89% yield) as a reddish liquid that solidified on standing. 1H NMR (300 MHz, DMSO-d6): δ ppm 9.12 (s, 1H), 7.63 (t, J=8.65 Hz, 1H), 7.42 (dd, J=10.85, 2.35 Hz, 1H), 7.18-7.24 (m, 1H), 1.45 (s, 9H). LCMS (Method 1): m/z 246 [M+H]+.
References:
US2016/83365,2016,A1 Location in patent:Paragraph 0648; 0649; 0650

24424-99-5
861 suppliers
$13.50/25G

956828-47-0
22 suppliers
$12.00/250mg