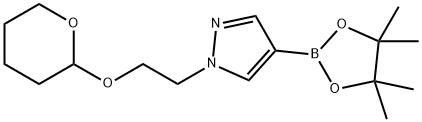
1-{2-[(Tetrahydro-2H-pyran-2-yl)oxy]ethyl}-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-{2-[(Tetrahydro-2H-pyran-2-yl)oxy]ethyl}-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:956907-34-9
- Molecular formula:C16H27BN2O4
- Molecular Weight:322.21
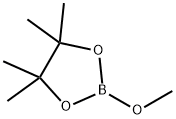
1195-66-0
182 suppliers
$5.00/1g
![1-{2-[(Tetrahydro-2H-pyran-2-yl)oxy]ethyl}-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole](/CAS/GIF/956907-34-9.gif)
956907-34-9
40 suppliers
$450.00/1 g
Yield: 80%
Reaction Conditions:
Stage #1:4-iodo-1-[2-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1H-pyrazole with isopropylmagnesium chloride in tetrahydrofuran at 0; for 1 h;
Stage #2:2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at 20; for 1 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran
Steps:
42.2
Step 2 To a solution of 4-iodo-1-[2-(tetrahydro-pyran-2-yloxy)-ethyl]-1H-pyrazole (1.0 g, 3.1 mmol) in anhydrous THF (8 mL) was added iPrMgCl (2M in THF, 3.10 mL, 6.21 mmol) at 0° C. drop by drop under nitrogen. The reaction was stirred for 1 hour at 0° C. under nitrogen. To the solution was added 2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (0.736 g, 4.66 mmoL) at 0° C. and the resulting yellow solution was allowed to stir for 1 hour at ambient temperature under nitrogen. The reaction was quenched with sat. aqueous solution of NH4Cl (10 mL). EtOAc (50 mL) and sat aqueous NH4Cl solution (10 mL) were added. The organic layer was separated, and the aqueous layer was extracted with EtOAc (3*50 mL), dried over Na2SO4, and concentrated to give the crude product as yellow oil. The oil was purified a silica gel column eluding with EtOAc and hexanes to provide 1-[2-(tetrahydro-pyran-2-yloxy)-ethyl]-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole as clear oil (800 mgs, 80% yield). 1H NMR (300 MHz, DMSO-d6) δ 7.91 (s, 1H) 4.48-4.54 (m, 1H) 4.26-4.33 (m, 2H) 3.86-3.90 (m, 1H) 3.66-3.76 (m, 1H) 3.45-3.57 (m, 1H) 3.33-3.39 (m, 1H) 1.33-1.70 (m, 6H) 1.24 (s, 12H). on a high vacuum for 1 hour. The solid was re-crystallized from EtOH (50 mL) to provide 2-[4-(3-Quinolin-6-ylmethyl-3H-[1,2,3]triazolo[4,5-b]pyrazin-5-yl)-pyrazol-1-yl]-ethanol (400, 63% yield) as a white crystalline solid with a melting point of 222° C. 1H NMR (400 MHz, DMSO-d6) δ 9.22 (s, 1H) 8.89 (dd, J=4.14, 1.70 Hz, 1H) 8.63 (s, 1H) 8.37 (dd, J=8.38, 1.04 Hz, 1H) 8.32 (s, 1H) 7.98-8.04 (m, 2H) 7.82 (dd, J=8.67, 2.07 Hz, 1H) 7.53 (dd, J=8.29, 4.14 Hz, 1H) 6.15 (s, 2H) 4.96 (t, J=5.27 Hz, 1H) 4.24 (t, J=5.46 Hz, 2H) 3.78 (q, J=5.46 Hz, 2H).
References:
PFIZER INC. US2007/265272, 2007, A1 Location in patent:Page/Page column 37; 39
![4-Iodo-1-[2-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1H-pyrazole](/CAS/GIF/879487-88-4.gif)
879487-88-4
15 suppliers
inquiry

1195-66-0
182 suppliers
$5.00/1g
![1-{2-[(Tetrahydro-2H-pyran-2-yl)oxy]ethyl}-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole](/CAS/GIF/956907-34-9.gif)
956907-34-9
40 suppliers
$450.00/1 g
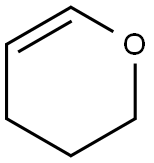
110-87-2
527 suppliers
$10.00/1g
![1-{2-[(Tetrahydro-2H-pyran-2-yl)oxy]ethyl}-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole](/CAS/GIF/956907-34-9.gif)
956907-34-9
40 suppliers
$450.00/1 g