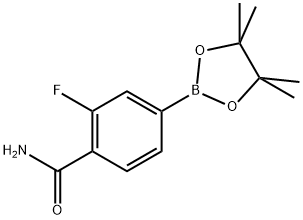
2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide synthesis
- Product Name:2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide
- CAS Number:957346-57-5
- Molecular formula:C13H17BFNO3
- Molecular Weight:265.09

292621-45-5
83 suppliers
$35.00/250mg
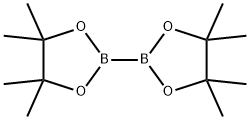
73183-34-3
570 suppliers
$6.00/5g

957346-57-5
12 suppliers
inquiry
Yield:957346-57-5 100%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 90; for 0.35 h;Microwave irradiation;
Steps:
E.1
E.1 2-Fluoro-4-(4, 4, 5, 5-tetramethyl-l, 3, 2-dioxaborolan-2-yl)benzamide[0360] Pd(dppf)Cl2 (21 mg, 0.028 mmol, 0.03 eq), potassium acetate (277 mg, 1.03 mmol, 2.82 mmol, 3 eq), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (263 mg, 1.1 eq), and 4-bromo-2- fluorobenzamide (205 mg, 0.94 mmol, 1.0 eq) are suspended in anhydrous dioxane (2 mL) in a 5-mL microwave vial and the mixture is purged with nitrogen for 1 min. The resulting slurry is heated for 20 min at 90 0C in a microwave (Biotage Initiator, Absorption high). DCM (2 mL) is added to the dark brown reaction mixture and the resulting suspension is filtered. The precipitate collected on the frit is washed with DCM (2 x 5 mL) and the solid residue remaining in the microwave tube is washed with DCM (2 x 2 mL). The DCM washes and filtrates are combined. Celite (1.5 g) is added to the organic extracts and the solvent is evaporated on a rotary evaporator to provide a free-flowing powder. The Celite/compound mixture was loaded onto a prepacked silica column (10 g of silica) and the column eluted with 1 :1 hexane/ethyl acetate to provide the crude 2-fluoro-4-(4,4,5, 5-tetramethyl-l, 3,2-
References:
WO2008/65198,2008,A1 Location in patent:Page/Page column 82-83
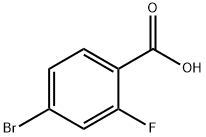
112704-79-7
513 suppliers
$15.00/5G

957346-57-5
12 suppliers
inquiry