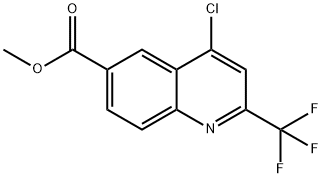
methyl 4-chloro-2-(trifluoromethyl)quinoline-6-carboxylate synthesis
- Product Name:methyl 4-chloro-2-(trifluoromethyl)quinoline-6-carboxylate
- CAS Number:958332-63-3
- Molecular formula:C12H7ClF3NO2
- Molecular Weight:289.64

958332-60-0
0 suppliers
inquiry

958332-63-3
13 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: methyl 4-hydroxy-2-(trifluoromethyl)quinoline-6-carboxylatewith trichlorophosphate at 110; for 20 h;
Stage #2: with ammonium hydroxide in water;Cooling with ice;
Stage #3: with hydrogenchloride in water; pH=5;
Steps:
63.63A
The above quinoline obtained was heated in neat POCl3 (20 mL) at 110° C. for 20 h. The reaction mixture was cooled and poured carefully into a mixture of NH4OH-ice water. The pH of the aqueous layer was acidified to pH 5 by adding 1N HCl and extracted with EtOAc. The combined organic layers were dried (MgSO4), concentrated in vacuo and the residue was triturated with MeOH/Et2O/hexane to give the title product as a beige solid (2800 mg, 32%).m/z=290.0 (M+1), r.t. 3.77 min.1H NMR (400 MHz; CD3OD) δ 8.91 (1H, d), 8.38 (1H, d), 8.19 (1H, d), 8.04 (1H, s), 3.93 (3H, s).
References:
US2012/88746,2012,A1 Location in patent:Page/Page column 74

619-45-4
381 suppliers
$6.00/1g

958332-63-3
13 suppliers
inquiry