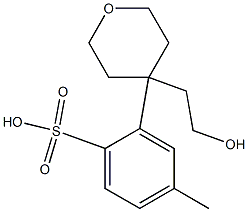
2-(4-(2-hydroxyethyl)-tetrahydro-2H-pyran-4-yl)-4-methylbenzenesulfonic acid synthesis
- Product Name:2-(4-(2-hydroxyethyl)-tetrahydro-2H-pyran-4-yl)-4-methylbenzenesulfonic acid
- CAS Number:959748-73-3
- Molecular formula:C14H20O4S
- Molecular Weight:284.37
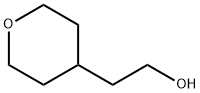
4677-18-3
119 suppliers
$26.00/100mg

98-59-9
583 suppliers
$9.00/5g

959748-73-3
18 suppliers
$177.21/1g
Yield:959748-73-3 53%
Reaction Conditions:
in pyridine at 20; for 5 h;
Steps:
D.2
To a solution of 1.63 g (12.5 mmol) of Compound 18 in pyridine (15 mL) are added 3.58 g (18.8 mmol) of p-toluenesulfonylchloride. The reaction is stirred at room temperature for 5 h. The reaction mixture is concentrated under reduced pressure. The residue is dissolved 2M aqueous HCl solution (20 mL) and extracted with ethyl acetate (3×50 mL). The combined organic extracts are dried over Na2SO4, filtered and the solvent is removed to give 1.9 g of Compound 19 as off-white crystalline solid. Yield: 53%; ES-MS: m/z 285 [M+H]; 1H-NMR (500 MHz, CHLOROFORM-d) δ ppm 1.17-1.29 (2H, m), 1.45-1.52 (2H, m), 1.57-1.67 (3H, m), 2.46 (3H, s), 3.32 (2H, td, J=11.78, 1.93 Hz), 3.91 (2H, dd, J=11.28, 4.13 Hz), 4.08 (2H, t, J=6.14 Hz), 7.36 (2H, d, J=8.07 Hz), 7.80 (2H, d, J=8.44 Hz).
References:
US2011/71196,2011,A1 Location in patent:Page/Page column 17-18
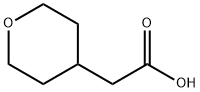
85064-61-5
147 suppliers
$6.00/100mg

959748-73-3
18 suppliers
$177.21/1g