
4-(4-(4-Methylpiperazin-1-yl)piperidin-1-yl)aniline synthesis
- Product Name:4-(4-(4-Methylpiperazin-1-yl)piperidin-1-yl)aniline
- CAS Number:959795-70-1
- Molecular formula:C16H26N4
- Molecular Weight:274.4
![4-[4-(4-Methyl-piperazin-1-yl)-piperidin-1-yl]-1-nitrobenzene](/CAS/20180703/GIF/959795-69-8.gif)
959795-69-8

959795-70-1
GENERAL METHOD: 1-methyl-4-(1-(4-nitrophenyl)piperidin-4-yl)piperazine (2.18 g, 6.52 mmol) was dissolved in ethanol (50 mL) and 10% palladium carbon (wet basis, 53% water; 600 mg) was added. The reaction mixture was stirred at room temperature and 1 atm hydrogen atmosphere for 8 hours. Upon completion of the reaction, the insoluble material was removed by Celite filtration and the filtrate was concentrated under vacuum to afford 4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]aniline (1.96 g, 99%) as a light purple solid.
![4-[4-(4-Methyl-piperazin-1-yl)-piperidin-1-yl]-1-nitrobenzene](/CAS/20180703/GIF/959795-69-8.gif)
959795-69-8
17 suppliers
inquiry

959795-70-1
66 suppliers
$22.00/100mg
Yield: 87%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 20; under 760.051 Torr; for 8 h;
Steps:
2-Methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]-aniline (5a)
General procedure: To a mixture of 1-[1-(3-methoxy-4-nitrophenyl)-piperidin-4-yl]-4-methylpiperazine 4a (2.18 g, 6.52 mmol) inEtOH (50 mL) was added 10% palladium on carbon (wet, contains53% water; 600 mg). The reaction mixture was stirred at room temperature for 8 h under 1 atm hydrogen atmosphere.The insoluble material was removed by filtration throughCelite, and the filtrate was concentrated in vacuo to give 5a (1.96 g, 99%) as a pale purple solid.
References:
Iikubo, Kazuhiko;Kondoh, Yutaka;Shimada, Itsuro;Matsuya, Takahiro;Mori, Kenichi;Ueno, Yoko;Okada, Minoru [Chemical and Pharmaceutical Bulletin,2018,vol. 66,# 3,p. 251 - 262]
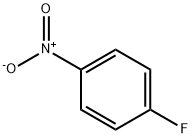
350-46-9
525 suppliers
$10.60/1gm:

959795-70-1
66 suppliers
$22.00/100mg

109-01-3
676 suppliers
$5.00/5 g

959795-70-1
66 suppliers
$22.00/100mg
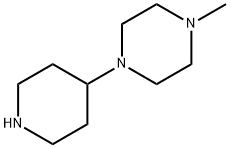
53617-36-0
209 suppliers
$8.00/1g

959795-70-1
66 suppliers
$22.00/100mg