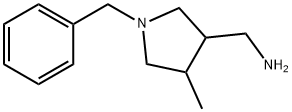
3-Pyrrolidinemethanamine, 4-methyl-1-(phenylmethyl)- synthesis
- Product Name:3-Pyrrolidinemethanamine, 4-methyl-1-(phenylmethyl)-
- CAS Number:959958-19-1
- Molecular formula:C13H20N2
- Molecular Weight:204.31
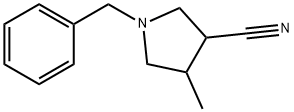
115687-24-6
8 suppliers
inquiry

959958-19-1
4 suppliers
inquiry
Yield:959958-19-1 39%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 25;
Steps:
AE.1
General Procedure AE: Reduction of a nitrile to an amineA flask is charged with a nitrile and an organic solvent such as THF, DCM, 1,4-dioxane, MeOH, toluene (preferably THF), and the mixture may be optionally cooled to about 0 to 10 °C. To the mixture is then added a reducing agent (such as LAH, Raney nickel /?, diborane, tin(II)chloride or cobalt(II)chloride). Optionally, the reducing agent can be added to the solvent followed by the cooling and then the nitrile is added. In cases where Raney nickel/H2 is used, NH3 or NH4OH may be added and water may be used as co-solvent. The mixture is stirred at about 25 to 80 °C (preferably rt) for about 2 to 20 h (preferably about 4 to 8 h). The mixture may optionally be concentrated in vacuo to give the target compound. Alternatively, the mixture is optionally filtered through a media (such as silica gel or Celite) which is rinsed with an appropriate solvent (such as EtOAc, 1,4-dioxane, THF, MeCN, DCM, Et20, MeOH, EtOH) and then optionally concentrated in vacuo to give a residue as the target compound. Either the residue or the solution may be optionally partitioned between water and an organic solvent (such as EtOAc, Et20 or DCM). The organic layer is isolated and may optionally be washed in no particular order with water and/or aqueous solutions containing an acid (such as HC1, AcOH or NH4C1) and/or aqueous solutions containing a base (such as NaHC03, Na2C03, NaOH, KOH or NH4OH) and/or aqueous solutions containing an inorganic salt (such as NaCl Na2S03 or Na2S203). The organic solution may then be optionally dried with a drying agent (such as anhydrous MgS04 or Na2S0 ), filtered and concentrated in vacuo to give the target compound.Illustration of General Procedure AE:Preparation No.AE.l (l-Benzyl-4-methylpyrrolidin-3-yl)methanamineTo a mixture of LAH (0.019 g, 0.499 mmol) in THF (5 mL) was added l-benzyl-4- methylpyrrolidine-3-carbonitrile (0.050 g, 0.250 mmol, Tyger) at about 0 °C, then the resulting mixture was stirred at about 25 °C for about 4 h. EtOAc (2 mL) and water (3 mL) were added and the precipitate formed was filtered off. The filtrate was concentrated to give (l-benzyl-4- methylpyrrolidin-3-yl)methanamine (0.05 g, 39 %): LCMS (Table 2 , Method 1) R, = 0.22 min.; MS m/z: 205 (M+H)+.
References:
WO2012/48222,2012,A1 Location in patent:Page/Page column 193-194