tert-butyl 6-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate synthesis
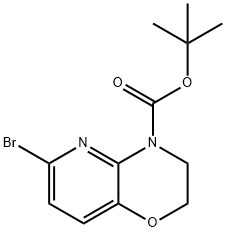
- Product Name:tert-butyl 6-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate
- CAS Number:959992-64-4
- Molecular formula:C12H15BrN2O3
- Molecular Weight:315.16
![6-BROMO-3,4-DIHYDRO-2H-PYRIDO[3,2-B][1,4]OXAZINE](/CAS/GIF/959992-62-2.gif)
959992-62-2
72 suppliers
$99.00/250mg

24424-99-5
861 suppliers
$13.50/25G
![tert-butyl 6-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate](/CAS/20180713/GIF/959992-64-4.gif)
959992-64-4
27 suppliers
$209.00/100mg
Yield:959992-64-4 97%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 0 - 40; for 3.25 h;
Steps:
Tert-Butyl-6-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylic acid methyl ester
Combine 6-bromo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine (1.86g, 8.65mmol), 4-dimethylaminopyridine (0.21g, 1.73mmol) ), triethylamine (1.75g, 17.3mmol) was added to dichloromethane (30mL), and the temperature was cooled to 0-10°C in an ice bath under the protection of nitrogen. BOC anhydride (2.83 g, 12.97 mmol) was slowly added to the reaction solution, stirred at room temperature for 15 minutes, and then heated to 40°C for 3 hours. The reaction solution was quenched by adding water and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and concentrated by column chromatography (petroleum ether/ethyl acetate=12/1) to obtain the titled white solid compound (2.64g, 97%)
References:
CN112979655,2021,A Location in patent:Paragraph 0747-0750

24424-99-5
861 suppliers
$13.50/25G
![tert-butyl 6-bromo-2H-pyrido[3,2-b][1,4]oxazine-4(3H)-carboxylate](/CAS/20180713/GIF/959992-64-4.gif)
959992-64-4
27 suppliers
$209.00/100mg