Yield:-
Reaction Conditions:
with caesium carbonate in acetonitrile at 20; for 1.5 h;
Steps:
24.1
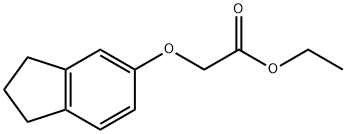
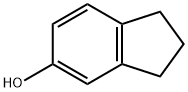
Example 24: N?(3-Fluoro-4-methanesulfonylaminobenzyl)-2-(indan-5- yloxy)acetamideStep 1 : Synthesis of indan-5-yloxyacetic acid; To a solution of 5-indanol (0.38 g, 2.83 mmol) in acetonitrile (10 mL) were added cesium carbonate (1.37 g, 4.2 mmol) and ethyl bromoacetate (0.34 mL, 3.01 mmol). The resulting mixture was stirred for 1.5 hours at room temperature, concentrated under reduced pressure, and diluted with EtOAc and water. The organic layer was washed with brine and concentrated under reduced pressure. The crude residue was diluted with THF (7 mL) and 1 N LiOH (5 mL), and then stirred for 1 hour at room temperature. The mixture was acidified with 6 N HCI, concentrated under reduced pressure, and diluted with EtOAc and water. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The crude residue was then purified by recrystallization from EtOAc/hexane to afford 433 mg (79.6%) as a white solid.1H NMR (300 MHz, CDCI3): δ 7.13 (d, 1 H, J = 8.1 Hz), 6.80 (d, 1 H, J = 2.1 Hz), 6.71 (dd, 1 H, J = 8.4, 2.7 Hz), 4.66 (s, 2 H), 2.88 (t, 2 H, J = 7.8 Hz)1 2.84 (t, 2 H, J = 7.2 Hz), 2.08 (quint, 2 H1 J = 7.2 Hz)
References:
WO2007/120012,2007,A1 Location in patent:Page/Page column 91-92