
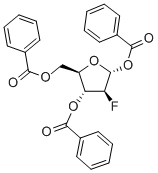
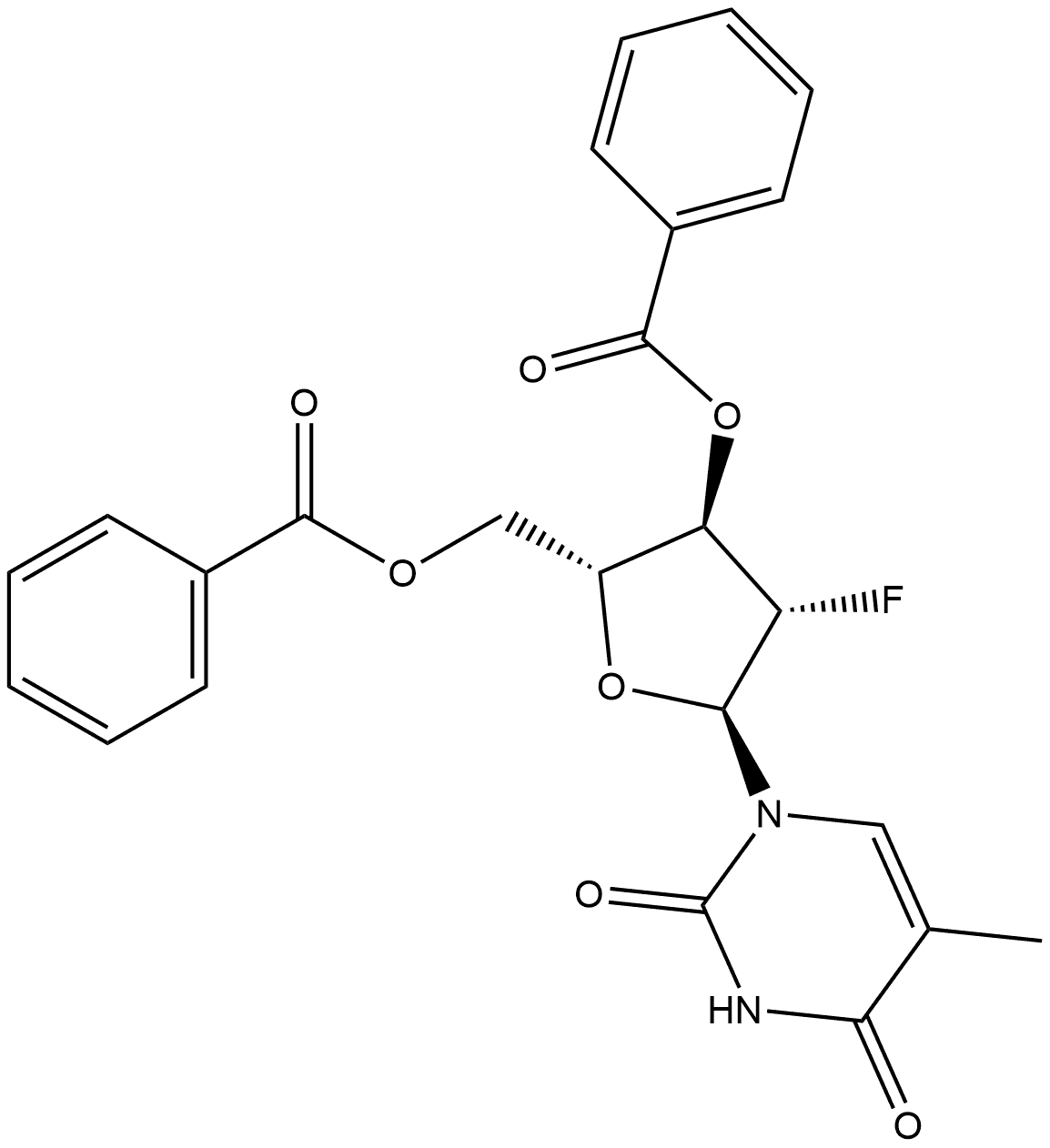
2,4(1H,3H)-Pyrimidinedione, 1-(3,5-di-O-benzoyl-2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-methyl- synthesis
- Product Name:2,4(1H,3H)-Pyrimidinedione, 1-(3,5-di-O-benzoyl-2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-methyl-
- CAS Number:97614-47-6
- Molecular formula:C24H21FN2O7
- Molecular Weight:468.4312
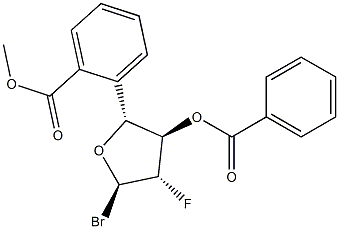
97614-44-3
66 suppliers
$45.00/100mg
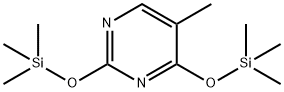
7288-28-0
59 suppliers
$60.00/250mg

97614-47-6
22 suppliers
inquiry
Yield:97614-47-6 70 g
Reaction Conditions:
in tetrachloromethane at 80; for 48 h;
Steps:
a.18 Preparation of compound 28-3:
To a solution of 28-2 (73 g, 0.173 mol) in CCl4(500 mL) was added 28a (70 g, 0.259 mol) at room temperature and the mixture was heated to 80oC and stirred at this temperature for 48 hours. The 28-2 was consumed and major desired product were detected by TLC and LC-MS. The reaction was poured to water (1000 mL) and extracted with DCM (300 mL *3). The combined organic layers were washed with (500 mL * 2), brine (1000 mL), dried over anhydrous Na2SO4and evaporated in thevacuo to give crude product, which was purified by column chromatography with a gradient of 10 to 35% EtOAc in PE to give 28-3 (70 g, 149.44 mmol, 86.6% yield) as white solid.1H NMR (400 MHz, CDCl3) δ 9.49 (s, 1H), 8.10 (dd, J = 16.7, 7.9 Hz, 4H), 7.72 - 7.57 (m, 2H), 7.49 (dt, J = 11.6, 7.7 Hz, 4H), 7.38 (s, 1H), 6.39 (dd, J = 22.1, 2.6 Hz, 1H), 5.66 (dd, J = 17.9, 2.6 Hz, 1H), 5.36 (dd, J = 50.2, 2.6 Hz, 1H), 4.83 (qd, J = 12.2, 4.0 Hz, 2H), 4.51 (d, J = 3.4 Hz, 1H), 1.77 (s, 3H). ESI-MS: m/z 469.0 [M+H]+.
References:
WO2021/119325,2021,A1 Location in patent:Paragraph 0056; 0333
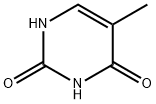
97614-43-2
333 suppliers
$5.00/250mg

97614-47-6
22 suppliers
inquiry
65-71-4
525 suppliers
$5.00/5g

97614-47-6
22 suppliers
inquiry

97614-43-2
333 suppliers
$5.00/250mg

65-71-4
525 suppliers
$5.00/5g

97614-47-6
22 suppliers
inquiry
97614-48-7
1 suppliers
inquiry
97614-42-1
41 suppliers
$55.00/10g

97614-47-6
22 suppliers
inquiry