
DMT-dA(bz) Phosphoramidite synthesis
- Product Name:DMT-dA(bz) Phosphoramidite
- CAS Number:98796-53-3
- Molecular formula:C47H52N7O7P
- Molecular Weight:857.93
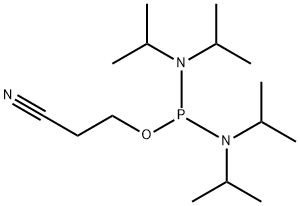
102691-36-1
368 suppliers
$6.00/1g
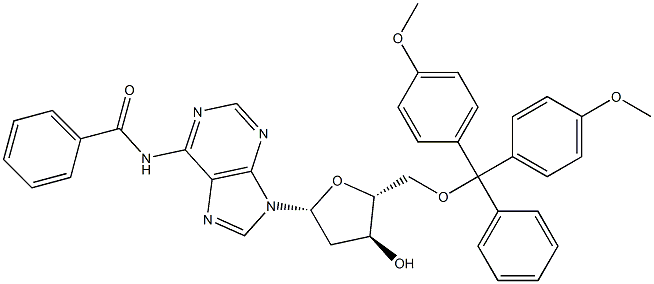
64325-78-6
176 suppliers
$9.00/250mg

98796-53-3
201 suppliers
$12.00/250mg
Yield:98796-53-3 96%
Reaction Conditions:
Stage #1: N,N,N',N'-tetraisopropyl 2-cyanoethylphosphorodiamidite;N6-benzoyl-5'-O-(4,4'-dimethoxytrityl)-2'-deoxyadenosine in acetonitrile at 20;
Stage #2: with 5-Phenyl-1H-tetrazole in acetonitrile at 20; for 2 - 24 h;Product distribution / selectivity;
Steps:
2; 3; 4
Example 2; Synthesis of 5'-O-(4,4'-dimethoxytrityl)-N6-benzoyl-2'-deoxyadenosine 3'-O-(2-cyanoethyl N,N-diisopropylphosphoroamidite) ; 2.0 g of 5'-O-(4,4'-dimethoxytrityl)-N6-benzoyl-2'-deoxyadenosine was mixed with 10 mL of dehydrated acetonitrile and 1.10 g of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite (1.2 equivalent to the molar number of a raw material) was dropped to a suspension stirred at a room temperature, followed by further stirring. Then, 0.53 g of 5-phenyl-1H-tetrazole (1.2 equivalent to the molar number of a raw material) was added thereto and the resulting mixture was stirred at a room temperature for 2 hours. The reaction solution was analyzed according to the high performance liquid chromatography (reverse phase column, eluent: water/acetonitrile 5/5 (TEAA 250 mM), detection wavelength: 254 nm). As a result, the yield was 96%. The reaction selectivity (HPLC area % of the entitled compound / HPLC area % of the by-product) represented by the ratio of the entitled compound to the by-product represented by the general formula [5c] was 152, [] wherein R1 represents a 2-cyanoethyl group; R4 represents a 4,4'-dimethoxytrityl group; R5 represents a hydrogen atom; and B represents N6-benzoyl-9-adenine. Further, the reaction solution after 96 hours was analyzed. As a result, the yield was 99% and the reaction selectivity was 187.; Example 3; Synthesis of 5'-O-(4,4'-dimethoxytrityl)-N6-benzoyl-2'-deoxyadenosine 3'-O-(2-cyanoethyl N,N-diisopropylphosphoroamidite); The reaction was conducted in the same manner as in Example 2, except that the equivalent of 5-phenyl-1H-tetrazole was changed. 0.04 g of 5-phenyl-1 H-tetrazole (0.1 equivalent to the molar number of a raw material) was used and the resulting mixture was stirred for 24 hours. The reaction solution was analyzed according to the high performance liquid chromatography (reverse phase column, eluent: water/acetonitrile 5/5 (TEAA 250 mM), detection wavelength: 254 nm). As a result, the yield was 99%. The reaction selectivity (HPLC area % of the entitled compound / HPLC area % of the by-product) represented by the ratio of the entitled compound to the by-product represented by the general formula [5c] was 699.; Example 4; Synthesis of 5'-O-(4,4'-dimethoxytrityl)-N6-benzoyl-2'-deoxyadenosine 3'-O-(2-cyanoethyl N,N-diisopropylphosphoroamidite) ; The reaction was conducted in the same manner as in Example 2, except that the equivalent of 5-phenyl-1 H-tetrazole was changed. 0.22 g of 5-phenyl-1 H-tetrazole (0.5 equivalent to the molar number of a raw material) was used and the resulting mixture was stirred for 8 hours. The reaction solution was analyzed according to the high performance liquid chromatography (reverse phase column, eluent: water/acetonitrile 5/5 (TEAA 250 mM), detection wavelength: 254 nm). As a result, the yield was 99%. The reaction selectivity (HPLC area % of the entitled compound / HPLC area % of the by-product) represented by the ratio of the entitled compound to the by-product represented by the general formula [5c] was 785.
References:
EP1582528,2005,A1 Location in patent:Page/Page column 9

124482-92-4
7 suppliers
inquiry

64325-78-6
176 suppliers
$9.00/250mg

98796-53-3
201 suppliers
$12.00/250mg
131420-63-8
0 suppliers
inquiry

64325-78-6
176 suppliers
$9.00/250mg

98796-53-3
201 suppliers
$12.00/250mg
![Adenosine, N-benzoyl-5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxy-, 3'-[N,N,N',N'-tetrakis(1-methylethyl)phosphorodiamidite]](/CAS/20210305/GIF/133728-84-4.gif)
133728-84-4
0 suppliers
inquiry

109-78-4
354 suppliers
$10.00/1g

98796-53-3
201 suppliers
$12.00/250mg

124482-92-4
7 suppliers
inquiry

98796-53-3
201 suppliers
$12.00/250mg