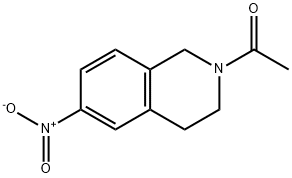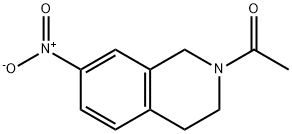
1-(3,4-dihydro-7-nitro-2(1H)-isoquinolinyl)ethanone synthesis
- Product Name:1-(3,4-dihydro-7-nitro-2(1H)-isoquinolinyl)ethanone
- CAS Number:99365-63-6
- Molecular formula:C11H12N2O3
- Molecular Weight:220.22
42923-79-5
124 suppliers
$15.00/250mg

75-36-5
560 suppliers
$17.92/100G

99365-63-6
9 suppliers
inquiry
Yield:99365-63-6 97%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20; for 1 h;
Steps:
49 1-(7-Nitro-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (49.1)
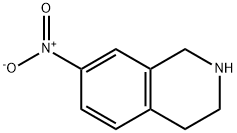
Acetyl chloride (1.3 g, 16.6 mmol) was slowly added to a solution of 7-nitro-1,2,3,4- tetrahydroisoquinoline (33.1) (2.0 g, 11.2 mmol) and triethylamine (2.3 g, 22.4 mmol) in THF (20 mL) at 0 °C, and the reaction mixture was stirred at ambient temperature for 1 h. The reaction mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate, and concentrated to afford the crude product. The crude product was purified by column chromatography (DCM/MeOH: 20/1) to afford 1-(7-nitro-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (49.1) as a brown solid (2.4 g, 97%). [00696] LCMS: 221.2 [M+1]+.
References:
WO2016/90079,2016,A1 Location in patent:Paragraph 00695-00696

42923-79-5
124 suppliers
$15.00/250mg

108-24-7
5 suppliers
$14.00/250ML

99365-63-6
9 suppliers
inquiry

91-21-4
253 suppliers
$5.00/5g

99365-63-6
9 suppliers
inquiry
![1-[3,4-Dihydroisoquinoline-2(1H)-yl]ethanone](/CAS/GIF/14028-67-2.gif)