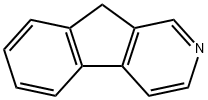
9H-Indeno[2,1-c]pyridine synthesis
- Product Name:9H-Indeno[2,1-c]pyridine
- CAS Number:244-40-6
- Molecular formula:C12H9N
- Molecular Weight:167.21
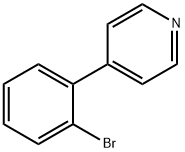
101681-34-9
33 suppliers
$45.00/2.5mg
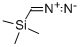
18107-18-1
207 suppliers
$33.00/5g
![9H-Indeno[2,1-c]pyridine](/CAS/GIF/244-40-6.gif)
244-40-6
2 suppliers
inquiry
Yield: 32%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium acetate;potassium carbonate in 1,4-dioxane;hexane at 100;Inert atmosphere;
Steps:
4.1.1. 2-((3-((4H-Indeno[1,2-b]thiophen-4-ylidene)methyl)pyridin-2-yl)oxy)ethan-1-amine (5)
General procedure: Thiophen-2-ylboronic acid (256 mg, 2.0 mmol) and Pd(PPh3)4(116 mg, 0.1 mmol) were added to an oven-dried Schlenk flask.After degassed with N2, 1-bromo-2-iodobenzene (622 mg,2.2 mmol), toluene (8 mL), ethanol (4 mL) and 1MNa2CO3 aqueoussolution (4 mL) were added and the reaction mixture was stirred at80 C overnight. After cooled to room temperature, 10 mL of Et2Owas added and the organic layer was then washed with saturatedbrine (10 mL) and dried over anhydrous Na2SO4. The solvent wasevaporated in vacuo and the resulting residue was purified by silicagel chromatography to give compound 2 as a colorless oil. Yield255 mg, 54%. Compound 2 (172 mg, 1.0 mmol), Pd(PPh3)4 (57 mg,0.05 mol), K2CO3 (138 mg, 1.0 mmol) and KOAc (98 mg, 1.0 mmol)were mixed in 10 mL of dioxane. After degassed with N2, trimethylsilyldiazomethane (2 N in hexane, 0.6 mL,1.2 mmol)was addedvia syringe. The mixture was stirred at 100 °C overnight and thenfiltered through celite. The solvents were evaporated in vacuo andthe residue was purified by silica gel chromatography to givecompound 4 as a pale solid. Yield 82 mg, 48%. To a solution ofcompound 4 (35 mg, 0.2 mmol) and tert-butyl (2-((3-formylpyridin-2-yl)oxy)ethyl)carbamate (53 mg, 0.2 mmol) in10 mL of methanol was added KF-Al2O3 (39 mg, 0.24 mmol). Thereaction mixture was stirred at 70 °C overnight, and then evaporatedin vacuo. The residue was purified by silica gel columnchromatography (ethyl acetate/CH2Cl2, 1:4) to give an intermediate,which was dissolved in CH2Cl2 (5 mL) and TFA (228 mg, 2.0 mmol)was slowly added. The reactionwas stirred at rt overnight and thentreated with saturated NaHCO3 (5 mL). The mixture was extractedwith CH2Cl2 (3 10 mL), dried over Na2SO4, filtered, concentratedand purified by silica gel column chromatography (CH2Cl2/MeOH)to give compound 5 as a pale yellow foam.
References:
Liu, Gang;Kim, Hyejin;Wang, Pingyuan;Fricke, Doerte R.;Chen, Haiying;Wang, Tianzhi;Shen, Qiang;Zhou, Jia [European Journal of Medicinal Chemistry,2021,vol. 219,art. no. 113427]
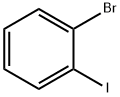
583-55-1
467 suppliers
$7.00/5g
![9H-Indeno[2,1-c]pyridine](/CAS/GIF/244-40-6.gif)
244-40-6
2 suppliers
inquiry
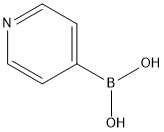
1692-15-5
444 suppliers
$6.00/1g
![9H-Indeno[2,1-c]pyridine](/CAS/GIF/244-40-6.gif)
244-40-6
2 suppliers
inquiry