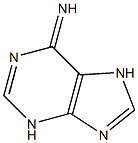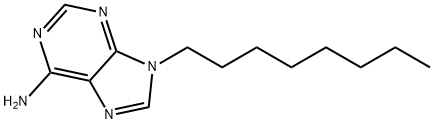
9H-Purin-6-amine, 9-octyl- synthesis
- Product Name:9H-Purin-6-amine, 9-octyl-
- CAS Number:728-35-8
- Molecular formula:C13H21N5
- Molecular Weight:247.34
Yield:728-35-8 79%
Reaction Conditions:
Stage #1: octanolwith phosphorus pentoxide;potassium iodide in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: adeninewith potassium carbonate;triethylamine in N,N-dimethyl-formamide; for 7 h;Reflux;regioselective reaction;Reagent/catalyst;Solvent;
Steps:
4.2 General procedure for synthesis of N-alkyl nucleobases via alcohols using P2O5/KI
General procedure: To a double-necked round bottom flask (100mL), equipped with a condenser, it was added a mixture of KI (1.5mmol), P2O5 (1.5mmol) and the desired alcohol (1mmol) in DMF (5mL). The reaction mixture was stirred at r.t. for 30min. Next, the considered nucleobase (1mmol), K2CO3 (1mmol) and Et3N (1mmol) were added and the reaction mixture was heated to reflux for a further 6.5-10.5h (until TLC indicated no further progress in reaction, Table5). The solvent was then evaporated at reduced pressure, and the remaining foam was dissolved in chloroform (150mL) and washed with water (2×150mL). The organic layer was dried on Na2SO4 (1g) and evaporated. The product was purified using short column chromatography on silica gel eluting with proper solvents.
References:
Behrouz, Somayeh;Soltani Rad, Mohammad Navid;Ahmadi, Samira [Tetrahedron,2019,vol. 75,# 36,art. no. 130499] Location in patent:supporting information