
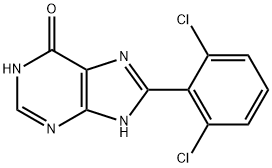
9H-Purine, 6-broMo-8-(2,6-dichlorophenyl)- synthesis
- Product Name:9H-Purine, 6-broMo-8-(2,6-dichlorophenyl)-
- CAS Number:1227958-56-6
- Molecular formula:C11H5BrCl2N4
- Molecular Weight:343.99
1227958-69-1
2 suppliers
inquiry

1227958-56-6
21 suppliers
$153.00/50mg
Yield:1227958-56-6 57%
Reaction Conditions:
with phosphorus(V) oxybromide in acetonitrile at 150; for 0.666667 h;
Steps:
216.1
To a 100 mL round bottom flask was charged 8-(2,6-dichlorophenyl)-9H-purin-6-ol(1.0 g, 3.56 mmol), followed by anhydrous acetonitrile (6.34 mL), POBr3 (3.34 g, 10.67 mmol) was added dropwise, and the mixture was heated at 150 °C for 40 min before being cooled to 23 °C. The mixture was carefully poured into ice water (20 mL), and then extracted with EtOAc (3 x 20 mL). Combined organics were dried over Na2S04, concentrated and purified by silica gel column chromatography (30% EtOAc/petroleum ether) to give pure 6- bromo-8-(2,6-dichloroφhenyl)-9H-purine (700 mg, 57% yield). *H NMR (DMSO-de, 400 MHz) δ 14.51 (s, 1H), 8.80 (s, 1H), 7.78-7.69 (m, 3H). LCMS(ESI) m/z: 344.9 [M+H+].
References:
WO2011/113802,2011,A2 Location in patent:Page/Page column 165
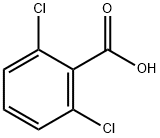
50-30-6
340 suppliers
$5.00/5g

1227958-56-6
21 suppliers
$153.00/50mg